Meteo 532 --
Atmospheric Chemistry -- Fall 2003
Final Examination
assigned:
01 December 2001
due: noon, Wednesday, 17 December 2001
Please keep your answers brief. As Strunk and White
say: “Omit needless words.” Please work
alone on this exam.
1. (20 points)
. It is said that globally the SO2 oxidation by gas-phase
reactions and by aqueous-phase reactions are comparable.
a) Estimate the fraction of the
troposphere (below 6 km) that would need to be occupied by clouds for this to
be true. Assume that most of the
oxidation occurs in the lowest part of the atmosphere, below 6 km, the average
pH is 5.0, [OH]midday =3x106 cm-3, H2O2
= 1 ppbv, and
O3 is not important in SO2 oxidation.
b) The SO2 flux into
the atmosphere is about 80 Tg (S) yr-1
. Estimate how many molecules of HOx
are consumed in yearly SO2 oxidation. Remember that the reaction HO2+HO2®HOOH+O2 is
the only significant HOOH atmospheric source.
2. (20 points) Give the evidence that the Antarctic ozone
hole results from chlorofluorocarbons.
Is quick remediation possible by either ozone replacement or chlorine
removal? Use numerical calculations of
the amount of ozone that is lost or the required amount of a chemical necessary
for chlorine removal to support your position.
For the following, provide explanations that are no more
than 250 words long. An equation counts
as a word. Pictures and figures may be
used. Each description is worth 15
points.
3. Using an ozone isopleth
diagram, explain why the EPA has been regulating only VOC emissions in an
effort to reduce O3 in urban areas.
4. Describe the
lifecycle of an aerosol that is created by gas-to-particle conversion.
5. Describe the
behaviors that low-vapor-pressure products of reactive organic gases can have
when interacting with preexisting aerosols.
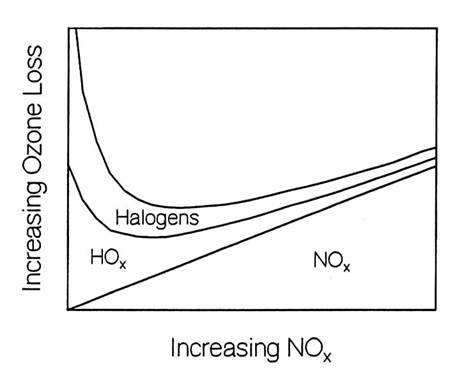
6. Explain
the chemical reasons for the variation of stratospheric ozone destruction by
halogen, HOx, and NOx catalytic
cycles as a function of increasing NOx, as
is presented schematically in the figure below.
Where would you put the Antarctic ozone hole on this figure?