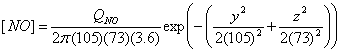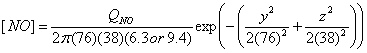Meteo 532 Atmospheric Chemistry
Fall 2003
Problem set 2†† Solutions
Problem 2.1. Energy and potentialtemperature.
Assume that we have a 1-kg air parcel at 70oN latitude. Use Figure
2.3 to do this problem. Why should you use the specific heat, constant
pressure, to do this problem?
††††††††††† a. How much
energy is required to raise it from 0 to 5 km?
††††††††††† b. How much
energy is required to raise it from 10 to 15 km?
††††††††††† c. What does
this tell you about the relative stability of the troposphere and ††††††††††††††††††††††††††††††††† ††††the stratosphere?
Solution.† We use cp because the air parcelís volume is not contained and can expand.† Futhermore, we are using potential temperature, which is the temperature that an air parcel would have at 1000 hPa.† The change in temperature is therefore just the change in potential temperature, and the energy required for the 1-kg parcel to go through from one potential temperature surface to another requires only that we know Dq and cp in the equation:† Ei-j = 1 kg (cp) Dq.
a.†
0-5 km.† Dq = 25
K, which mean E0-5 = 1 kg x 1040 J K-1 kg-1 x
25 K = 26 kJ
b. 10-15 km.† Dq = 80 K, so that E10-15 = 1 kg x 1040 J K-1 kg-1 x 80 K = 83 kJ
c.†
Because it requires more than 3 times the energy to raise a 1-kg
air parcel 5 km in the stratosphere compared to the troposphere, and because
the local source of heating at 10 km is so much smaller than the local source
of heat at 0 km, the stratosphere must be much more stable than the
troposphere.
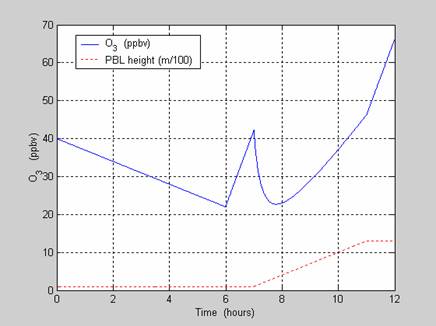
Problem 2.2.† A simplified morning rush
hour problem.† O3ís
mixing ratio at the surface is influenced not only by the chemical production
and loss of ozone, but also by the height of the planetary boundary layer.††† The net production of ozone can be
approximated by the following function: from
††††††††††† a.† Using a computer
program or Excel, plot O3ís surface mixing ratio in ppb.† †††††††††††††††††††††††††††† Assume
that there is no horizontal advection (i.e., no wind).
MATLAB m-file program called o3calc.m
% o3calc.m finds and plots the time-dependent O3 change due
to chemical
% production and boundary layer growth.
%
h0 = 100;
o30 = 40;
dt = 0.5/60;
% I choose 0.5-minute intervals for the time step.
% At each 0.5-minute step, we add
the chemically produced ozone to the existing ozone
% and then dilute the ozone by the ratio of the growth of
the PBL height from
% the begining
to the end of the 5-minute interval.
%
% We are assuming that ozone is
being produced throughout
% the PBL as it grows.
%
%
o3(1) = o30 - 3*dt;
%
%† For
%
for i= 2:719
††† t(i) = i/120;
††† h(i) = h0;
††† o3(i) = (o3(i-1) - 3*dt)*(h0./h(i));
end
%
% For
%
for i= 720:839
††† t(i) = i/120;
††† h(i) = h0;
††† o3(i) = (o3(i-1) + 20*dt)*(h(i-1)./h(i));
end
%
% For
%
for i= 840:1320
††† t(i) = i/120;
††† h(i) = h0 + 300*(t(i)-7);
††† o3(i) = (o3(i-1) + 20*dt)*(h(i-1)./h(i));
end
%
% For 11 am to
%
for i= 1321:1440
††† t(i) = i/120;
††† h(i) = h(1320);
††† o3(i) = (o3(i-1) + 20*dt)*(h(i-1)./h(i));
end
%
plot(t,o3,t,h/100,'r:')
xlabel('Time† (hours)')
ylabel('O_3†† (ppbv)')
legend('O_3† (ppbv)','PBL height
(m/100)')
grid††††††
†††††††
†††††† †
Problem 2.3. Dispersion
in a Gaussian plume. Using thePasquill turbulence
types and the Briggs formulae, estimate the Gaussian plume expansion for the
conditions at
|
|
|
|
|
|
|
|
time |
wind speed (m/s) |
conditions |
Pasquill type |
sy (m) @ 1 km |
sz (m) @ 1 km |
|
††††††††††††††††††††††††††
|
3.6 |
mostly overcast; slight insolation |
C |
105 |
73 |
|
|
6.3 |
overcast; slight insolation |
D |
76 |
38 |
|
|
9.4 |
overcast |
D |
76 |
38 |
I use the Briggs equations to calculate the dispersion in the y and z directions.† I assume rural conditions.†
|
|
sy† (m) |
sz† (m) |
|
C |
0.11 x (1 + 0.0001 x)-1/2 |
0.08 x (1 + 0.0002 x)-1/2 |
|
D |
0.08 x (1 + 0.0001 x)-1/2 |
0.06 x (1 + 0.0015 x)-1/2 |
At