Meteo 532 Atmospheric Chemistry
Problem set #4 – Solutions
Fall 2003
4.1
Tropospheric ozone
production and loss.
a) Write down the expression for the net tropospheric ozone production:
d[O3]/dt = P-L=? Remember to include the terms that destroy ozone as well as the ones that make ozone. Assume that RO2 is negligible.
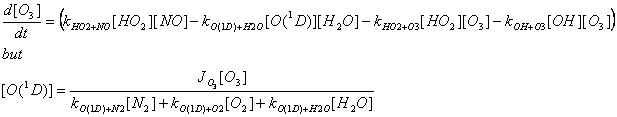
b) We know that ozone is actually produced from NO2+hv®NO+O, followed by O+O2+M®O3+M. Why does the expression for d[O3]/dt contain no terms for these two reactions?
Ozone production is from the two reactions listed above. However, they also participate in a reaction sequence that creates no new ozone – the NOx photostationary state. In this case, these two reactions balance the destruction of ozone by NO+O3 ® NO2+O3. Thus, all the production and destruction of ozone that is not balanced in the NOx photostationary state must pass through the reactions in the rate equation in a). We could list the rate equation with the two reactions listed above, but then we would need to realize that a lot of the cycling was simply the null cycle of the NOx photostationary state.
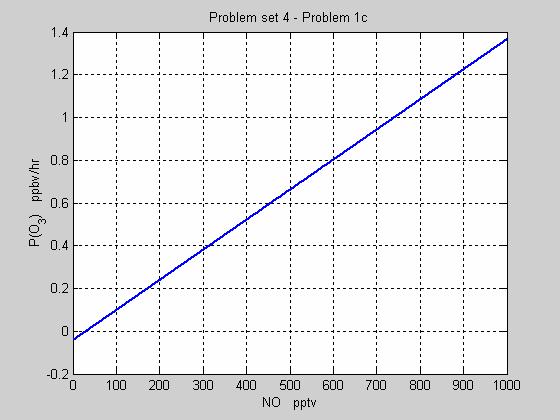
c) Plot the net ozone production rate, in ppb hr-1, at 10 km altitude as a function of NO from 1 to 1000 pptv for HO2=5 pptv, OH=0.5 pptv, O3=60 ppbv, H2O=200 ppmv, and JO3=2x10-5 s-1? Assume also the p=250 mb and T=235 K and that [RO2] is negligible.
% m532_ps4_p1.m calculates ozone net production and plots it
% as a function of NO
%
% Find the number density at 10 km.
T = 235;
p = 250;
M = 2.69e19*(273/T)*(p/1013);
%
% Establish the rate coefficients
%
JO3 = 2e-5;
k_ho2_no = 3.5e-12*exp(250/T);
k_o1d_h2o = 2.2e-10;
k_o1d_n2 = 1.8e-11*exp(110/T);
k_o1d_o2 = 3.2e-11*exp(70/T);
k_ho2_o3 = 1.0e-14*exp(-490/T);
k_oh_o3 = 1.7e-12*exp(-940/T);
%
% Set up a vector for NO between 1 and 1000 pptv
for i=1:1000
NO(i)=i;
end
%
% Find the concentrations of the trace chemicals
HO2_c = 5e-12*M;
OH_c = 0.5e-12*M;
O3_c = 60e-9*M;
H2O_c = 200e-6*M;
NO_c = NO*1e-12*M;
N2_c = 0.79*M;
O2_c = 0.20*M;
%
% Solve the rate equation
%
f = (k_o1d_h2o*H2O_c)/(k_o1d_h2o*H2O_c+k_o1d_n2*N2_c+k_o1d_o2*O2_c);
PO3 = (k_ho2_no*HO2_c*NO_c)-(JO3*O3_c*f+k_ho2_o3*HO2_c*O3_c+...
k_oh_o3*OH_c*O3_c);
PO3_ppbhr = (1e9/M)*PO3*3600;
%
% Plot PO3 as a function of NO
plot(NO, PO3_ppbhr,'linewidth',2)
grid
xlabel('NO pptv')
ylabel('P(O_3) ppbv/hr')
title('Problem set 4 - Problem 1c')
d) Consider the set of reactions:
OH+CO®H+CO2
H+O2+M®HO2+M
OH+O3®HO2+O2
HO2+NO®OH+NO2
HO2+O3®OH+2O2
Write down the rate equation for OH, assuming that the second reaction is much faster than the others.
![]()
e) Assuming that OH and HO2 are in steady-state, write down the [HO2]/[OH] ratio in terms of [NO], [CO], [O3], and the rate coefficients.
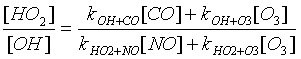
4.2 Variation of ozone
production with [NO].
HOx, the sum ofOH+HO2, has the rate equation:
d[HOx]/dt=P(HOx)–{2kHO2+HO2[HO2][HO2]+2kOH+HO2[OH][HO2]
+kOH+NO2+M[M][NO2][OH]}
where P(HOx) is the production rate (molecules cm-3s-1)
of
Assume that [HOx] approximately equals [HO2],
that OH, HO2, and thus HOx are in steady-state, that the
relationship that you derived for[HO2]/[OH] in problem 4.1, section
e applies, and that P(HOx) is 107molecules cm-3s-1,
COis 100 ppbv, O3 is 40 ppbv initially, p = 1000 hPa, T=298 K, and
RO2 is negligible.
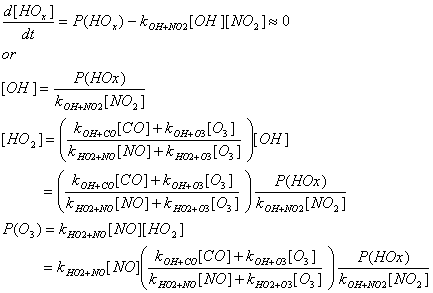
Using the equations in problem 4.1, section d, determine the analytical
expressions for [OH],[HO2], and P(O3)
in terms of P(HOx), [NO], [CO],[O3], and rate
coefficients for the three NO regimes. For this problem, P(O3)
=kNO+HO2[NO] [HO2].
Low-NOx regime – the main loss is HO2 + HO2 ® HOOH + O2.
Thus,
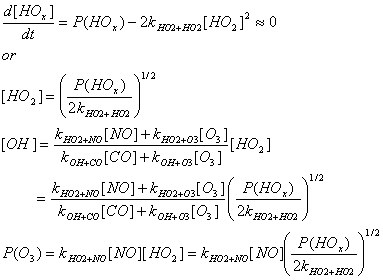
Mid-NOx regime – the main loss is OH + HO2 ® H2O + O2.
Thus,
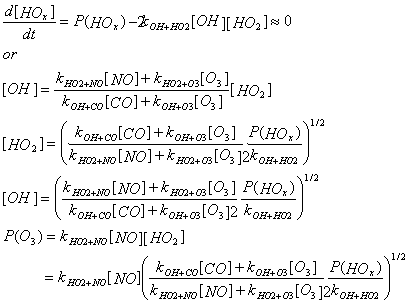
High NOx Regime – The main loss is by OH + NO2 + M ® HNO3 + M
We see that in the low and mid NOx regimes, [OH], [HO2], and P(O3) all depend on the square root of P(HOx), while in the high-NOx regime, they all depend linearly on P(HOx).
For most cases, the reaction of HO2 with NO is
much greater than its reaction with O3, even in much of the low-NOx
regime. If we assume this is the casem
then in the low NOx regime, both [OH] and P(O3)
depend roughly linearly with NO, while [HO2] is independent of
NO. In the mid-NOx regime,
[HO2] depends roughly on the inverse square root of NO, while [OH]
and P(O3) depend on the square root of
NO. In the high-NOx regime,
[HO2] depends roughly on the inverse
4.3.
Ozone production from the imbalance in the NOx photostationary
state.
Consider the [NO2]/[NO] relationship given above – the actual photochemical
state (APS), and compare it to the NOx photostationary state
relationship (PSS). Prior to the ability
to measure the oxidants HO2 and CH3O2,
researchers tried to determine the sum of [HO2]+[CH3O2]
by looking at the deviation of the APS from the PSS, where [NO], [NO2],
JNO2, and [O3] were all measured. They assumed that kHO2+NO and kCH3O2+NO
were the same.
a) Derive the equation for [HO2]+[CH3O2] in terms of the equation
just above

b) For T=298 K, p=1013 hPa, JNO2 =
8 x 10-3 s-1, O3=60 ppbv, and NOx =
1ppbv, what is the ratio [NO2]/[NO]
according to the PPS relationship?
For these conditions, [M]=2.46x1019
cm-3, [O3] = 60x10-9 2.46x1019 =
1.48x1012 cm-3;
[NOx] = [NO] + [NO2] =
10-9 2.46x1019 = 2.46x1010 cm-3.
![]()
c) If we include 10 pptv of both HO2
and RO2, what is the deviation of the APS from the PSS, in the form:
dev = ([NO2]/[NO]APS - [NO2]/[NO]PSS)?
For APS, kHO2+NO = 8.1x10-12 cm3
molecule-1 s-1.
[HO2] = 2.46x108 cm-3.
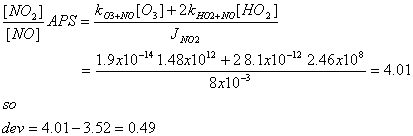
d) Measurements have uncertainties: for NO,
7%; for NO2, 10%; for O3, 3%; for kNO+O3, 10%;
and for JNO2, 5%. The
measurement uncertainty can be found by taking the expression:
err = [NO2]/[NO] – kNO+O3 [O3]/JNO2,
taking the derivative of err with respect to the 5 parameters; taking the
square root of the sum of the squares of the resulting terms. (Note: you can easily get each term in the
derivative into the form ¶A/A.
For example: ¶[NO2]/[NO] = (¶[NO2]/[NO2])([NO2]/[NO]).) How does the deviation from c) compare to the
measurement uncertainty? What does this
tell you about this measurement technique for [HO2] + [RO2]?
Putting these fractional errors in absolute terms,
[NO2] = (3.51/(1+3.51))2.46x1010
= 1.91x1010 cm-3
[NO] = (1/(1+3.51)) 2.46x1010 = 5.45x109
cm-3.
D[NO] = 0.07 5.45x109 = 3.8x108
cm-3
D[NO2] = 0.10 1.91x1010
= 1.91x109 cm-3
D[O3] = 0.03 1.48x1012
cm-3 = 4.44x1010 cm-3
DkNO+O3 = 0.10 1.9x10-14 = 1.9x10-14 cm3
molecule-1 s-1
DJNO2 = 0.05 8x10-3 =
4x10-4 s-1
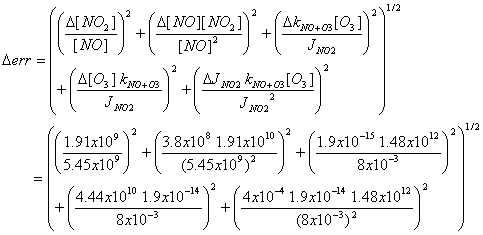
Derr = 0.59
Thus Derr is very similar to the deviation that we expect from the NOx photostationary state. This suggests that using the deviation from the NOx photostationary steady state to determine the instantaneous ozone production, or the concentrations of HO2 and RO2, is very difficult and not statistically significant.
4.4 Chlorine
oxidation of hydrocarbons
As pollutants from the urban centers on the East Coast are
swept out over the
Some reactions on surfaces have been observed to convert the chlorine in NaCl into gaseous Cl2. The main chemical loss for Cl2 is photolysis into 2 chlorine atoms. The chlorine atoms, Cl, can then react with hydrocarbons. For this problem, we chose ethane, C2H6, as the hydrocarbon. The beginning of this reaction sequence can be described by the reactions:
Cl2 + hn ® 2 Cl Ja (ra)
C2H6 + Cl ® C2H5 + HCl kb (rb)
C2H6 + OH ® C2H5 + H2O kc (rc)
The temperature is 298 K.
The pressure is 1013 hPa. For
these conditions, kb = 5.7 x 10-11 cm3
molecule-1 s-1 and kc = 2.4 x 10-13
cm3 molecule-1 s-1. Assume that the C2H6
mixing ratio is 10 ppbv, and that [OH] = 3 x 106 molecules cm-3. The photolysis frequency for Cl2
is 1.6 x 10-3 s-1.
[M] = 2.46x1019 cm-3 implies that [C2H6] = 2.46x1011 cm-3.
a) If the mixing ratio of C2H6 is 10 ppbv, what are the lifetimes of OH and Cl?
tOH = (kc [C2H6])-1 = 17 s
tCl = (kb [C2H6])-1 = 0.071 s
b) We want to know if Cl is an important oxidant in the marine environment. What Cl concentration is required for Cl to oxidize ethane as fast as OH does?
To determine the Cl concentration that will have the same C2H6 destruction rate as OH, we multiply [OH] by the ratio of the OH reaction rate coefficient to the Cl reaction rate coefficient.
This value of [Cl]equivalent = (2.3x10-13/5.7x10-11) 3x106 = 1.2 x 104 cm-3
c) What is the expression for the steady-state concentration of Cl?
Assume that we have 10 pptv of Cl2
![]()
d) The observed mixing ratio of Cl2 in one experiment is about 50 pptv near midday. With this source of Cl, what fraction of the oxidation of ethane is by Cl and what fraction is by OH? Is Cl an important oxidant?
OH: 2.3x10-13 3 x 106 = 6.9 x 10‑7 s-1
Cl: 5.7x10-11 2.8x105 = 1.60 x 10-5 s-1
total: 1.665 x 10-5 s-1.
96% is by Cl and 4% is by OH. Cl can be an important oxidant in the marine boundary layer.
e) What is the lifetime of C2H6 in the marine
environment, assuming that the diurnal average of [OH] and [Cl] are 1/3 of
their
tC2H6 = (1.665x10-5/3)-1 = 1.80x105 s = 2.1 days