What are
methane hydrates? 
In the search
for fuels that are alternative to petroleum and plentiful, a lot of recent
talk has centered on what are called methane hydrates (also called methane
clathrates or methane ice). Essentially, these are chunks of ice that are
saturated with methane or natural gas (during formation, the gas becomes
trapped in the structure of the freezing water). Because of their frozen
structure, they are to be found in arctic permafrost regions or at the
bottom of the ocean in places where conditions are favorable.
As with
natural gas, methane hydrates can be formed through biogenic or thermogenic
processes:
In biogenic
formation, the methane is produced by biological activity as
microorganisms attempt to decompose the remains of marine life (as above,
primarily marine phytoplankton and zooplankton). In this case, methane is
produced by the anoxic behaviors of methanogenic bacteria.
In thermogenic
formation, the gas is formed in the same manner as natural gas…through
catagenesis of kerogen. In fact, this may be the same natural gas that was
formed above, it just migrates to a region (remember, gas is 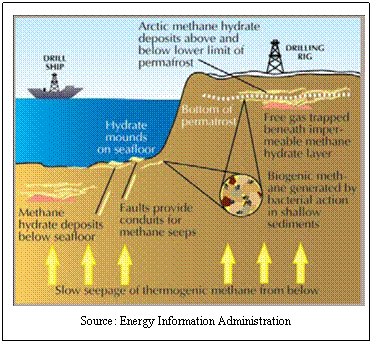 lighter
than earth materials and wants to reach the surface) where the formation of
hydrates is favorable. lighter
than earth materials and wants to reach the surface) where the formation of
hydrates is favorable.
Through
either method of formation (biogenic or thermogenic) the gases, once
formed, are thought to migrate (perhaps through geologic faults) and, upon
contact with cold sea water, to crystallize into ice-like structures.
Historically,
the U.S. has been able to produce roughly the same quantity of natural gas
that it needs to supply energy to the country (about 23% of countrywide
energy use). However, estimates project an increase of 18% in the amount of
natural gas that will be required in 2030. Therefore, it is becoming increasingly
important to locate further sources of natural gas, and methane hydrates
could fill this need. Hydrates are projected to exist in huge quantities
(approximately the amount energy contained in all the world’s other fossil
fuels combined…although, as we have yet to develop a successful extraction
method, the fraction of this that is accessible remains an open question).
The
difficulty arising in the utilization of methane hydrates owes primarily to
the development of an efficient extraction process. Beneath the sea floor,
hydrates are stable (at these pressures, the ice chunks will remain in
their structures up to about 18 degrees Celsius), but once removed, the ice
become unstable as pressures decrease and the gas develops a strong desire
to escape. Methods for efficient extraction are currently in development.
|