How do we use the petroleum?
 To
be of use to us, the crude oil must be “fractionated” into its various
hydrocarbons. This is done at the refinery. To
be of use to us, the crude oil must be “fractionated” into its various
hydrocarbons. This is done at the refinery.
Oil can be used in many different products, and this is
because of its composition of many different hydrocarbons of different
sizes, which are individually useful in different ways due to their
different properties. The purpose of a refinery is to separate and purify
these different components. Most refinery products can be grouped into
three classes: Light distillates (liquefied petroleum gas, naphtha, and
gasoline), middle distillates (kerosene and diesel), and heavy distillates
(fuel oil, lubricating oil, waxes, and tar). While all of these products
are familiar to consumers, some of them may have gained fame under their
refined forms. For instance, naphtha is the primary feedstock for producing
a high octane gasoline component and also is commonly used as cleaning
solvent, and kerosene is the main ingredient in many jet fuels.
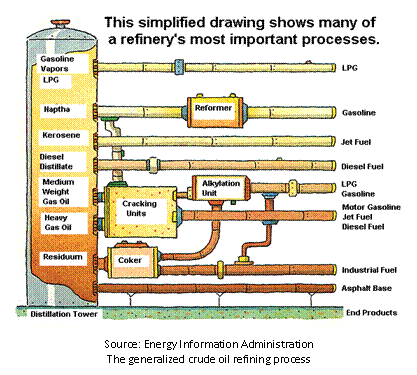
In a refinery, components are primarily separated using
“fractional distillation”. After being sent through a furnace, the crude
petroleum enters a fractionating column, where the products condense at
different temperatures within the column, so that the lighter components separate
out at the top of the column (they have lower boiling points than heavier
ones) and the heavier ones fall towards the bottom. Because this process
occurs at atmospheric pressure, it may be called atmospheric distillation.
Some of the heavier components that are difficult to separate may then
undergo vacuum distillation (fractional distillation in a vacuum) for
further separation. The heaviest components are then commonly “cracked”
(undergoing catagenesis) to form lighter hydrocarbons, which may be more
useful. In the same manner that natural mineral catalysts help to transform
kerogen to crude oil through the process of catagenesis, metal catalysts
can help transform large hydrocarbons into smaller ones. The modern form of
“catalytic cracking” utilizes hydrogen as catalyst, and is thus termed
“hydrocracking”. This is a primary process used in modern petroleum
refining to form more valuable lighter fuels from heavier ones. All of the
products then undergo further refinement in different units that produce
the desired products.
Alkanes are saturated hydrocarbons with between 5 and 40
carbon atoms per molecule which contain only hydrogen and carbon. The light
distillates range in molecular composition from pentane (5 carbons: C5H12)
to octane (8 carbons: C8H18). Middle distillates
range from nonane (9 carbons: C9H20) to hexadecane
(16 carbons: C16H34) while anything heavier is termed
a heavy distillate. Hydrocarbons that are lighter than pentane are
considered natural gas or natural gas liquids (liquefied petroleum gas).
![Text Box:
Source: Energy Information Administration
2006 U.S. average production of refined petroleum products (total = 6.56 billion barrels of oil)
[distillate fuel oil includes heating oil and diesel fuel]
[liquid refinery gasses include ethane/ethylene, propylene, butane/butylenes, and isobutene/isobutylene]](Petroleum_2_files/image013.gif) A
few further refinement processes are described below: A
few further refinement processes are described below:
·
Desalting removes salt from crude oil
before entering fractional distillation.
·
Desulfurization removes sulfur from compounds,
and several methods are possible. Hydrodesulfurization is the typical
method, and uses hydrogen to extract the sulfur. This occurs after
distillation.
·
Cracking breaks carbon-carbon bonds
to turn heavier hydrocarbons into lighter ones. This can occur thermally
(as occurs during the petroleum formation process beneath the earth) or
through the action of a catalyst:
o
Thermal
Cracking
§ Steam, visbreaking, or
coking
o
Catalytic
cracking
§ Fluid catalytic cracking (FCC) cracks heavy oils
into diesel and gasoline. Uses a hot fluid catalyst.
§ Hydrocracking (similar to FCC but lower
temperature and using hydrogen as catalyst) cracks heavy oils into gasoline
and kerosene
·
A
catalytic reformer converts naphtha into a higher octane form, which
has a higher content of aromatics, olefins, and cyclic hydrocarbons.
Hydrogen is a byproduct, and may be recycled and used in the naphtha
hydrotreater.
·
Steam
reforming
is a method of producing hydrogen from hydrocarbons, which may then be used
in other processes.
·
Solvent
dewaxing
removes heavy wax constituents from the vacuum distillation products.
See
this very nice animation of distillation and good tutorial of the refining
process. http://science.howstuffworks.com/oil-refining2.htm
|