COAL STRUCTURE
Coals of different age have different chemical compositions, and therefore different structures. Even within a certain age group (or rank) of coal, such as lignites or bituminous coals, the structure may vary depending on the environment in which a particular coal was formed.
Being solid, coals have a high molecular weight, much higher than that of natural gas or petroleum. Remember that natural gas is mostly methane (whose molecules contain one carbon atom surrounded by four hydrogen atoms, with a molecular weight of 16 grams per mole) and that a typical constituent of gasoline is octane (eight carbon atoms surrounded by eighteen hydrogen atoms, with a molecular weight of 114). In that sense, coals are somewhat similar to polymers (main constituents of plastics). A typical structure is illustrated below, where the hexagons (and occasional pentagons) represent six (or five) carbon atoms arranged in the so-called aromatic structure.
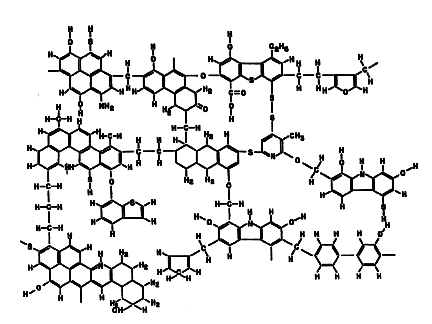
Compare this with the structures of some familiar but less complex organic materials, such as aspirin or cholesterol.