
The vast majority of fossil fuels is burnt with oxygen from air to produce heat. [Heat is either the ultimate objective or it is subsequently used in a heat engine (for example, turbine of a power plant or automobile engine) to produce mechanical work.] This process is called combustion. It is the most important chemical reaction known to man. The molecules of fossil fuels (for example methane, CH4, consisting of one carbon atom bound by four hydrogen atoms) react with the oxygen molecules (O2, two oxygen atoms connected to each other) and a rearrangement of their constituent atoms takes place. When the reaction is completed, carbon dioxide (CO2, one carbon atom bound by two oxygen atoms) and water (H2O, one oxygen atom bound by two hydrogen atoms) are produced. A consequence of this rearrangement of atoms is a release of heat. The quantity of heat released is called the heating value of the fuel. For example, a gallon of most petroleum products (gasoline, fuel oils, etc.) has a heating value of approximately 140,000 BTU. A barrel of oil (42 gallons) releases about 5.8 million BTU. A pound of coal has a heating value anywhere between 6,000 and 15,000 BTU. A cubic foot of natural gas has a heating value of about 1,000 BTU. (A useful rule of thumb, sufficiently accurate for many purposes, is that 1 gallon of oil is equivalent to about 10 pounds of coal and to about 150 cubic feet of natural gas. When these quantities of fuel are burnt, approximately the same quantity of energy is released in the form of heat.)
We shall see that in nuclear reactions, in contrast to this rearrangement of atoms to form new molecules, the internal constituents of the nucleus of an atom (protons and neutrons) are rearranged. Such a rearrangement also results in the release of heat, a tremendous amount of heat in fact. To give you an idea, a quarter-inch by half-inch pellet of conventional nuclear fuel (uranium) produces as much heat as 1800 pounds of coal, or 150 gallons of oil.
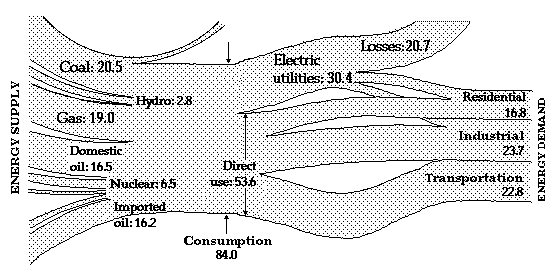
Because fossil fuels are of interest for their heating value, a very important comparison of their virtues is in terms of cost of a given quantity of thermal energy. A convenient choice is a million British thermal units (BTU). While the prices of fuels may vary considerably with time and place of purchase, the procedure outlined below is valid always. It allows us to compare 'apples' with 'apples'. It is also the key to formulating energy policies at both the microeconomic and the macroeconomic level.

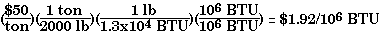
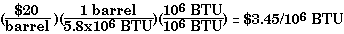
For an illustration of current prices of fossil fuels, as reported by the media, see here.