MatSE 259 - Second exam Fall 2005
Put student number on answer sheet
Only one correct answer for each question
Turn in exam sheet and answer sheet when finished
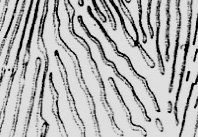
Match the descriptions in 1-4 with the microstructures in the images on the next page
1. Martensite
a.
b.
c.
d.
e.
2. Hypereutectoid steel
a.
b.
c.
d.
e.
3. Hypoeutectoid steel
a.
b.
c.
d.
e.
4. Pearlite
a.
b.
c.
d.
e.
a.

b.
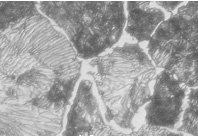
c.

d.
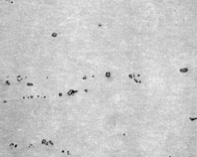
e.
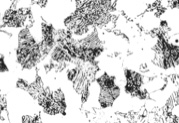
5. Which will shift TTT curves for steel to the left?
a. rapid quenching
b. add carbon
c. add alloy elements
d. decrease AUS grain size
e. none of the above
6. Which of the following love carbon the most?
a. Dr. Ryba's atoms
b. iron carbide (Fe3¬C) atoms
c. iron atoms
d. chromium atoms
7. Which of the following is a true statement:
a. MAR in steels is a metastable, non-equilibrium phase
b. under equilibrium conditions, MAR can be formed by quenching a pearlitic steel from any temperature below 700° C as long as it is quenched through the ms temperature
c. a martensitic steel must be tempered in order to form the austenite phase
d. tempering MAR for long periods at high tempering temperatures causes P to form
e. tempering MAR for long periods at high tempering temperatures causes proeutectoid CM to form
8. Estimate the strength of a 1060 steel heated treated so as to contain the equilibrium phases:
a. 40 ksi
b. 60 ksi
c. 80 ksi
d. 100 ksi
e. 120 ksi
9. Purpose of martempering
a. produce tempered MAR
b. form MAR above the ms temperature
c. avoid quench cracking
d. raise the ms
e. lower the ms
10. We discussed a number of reasons why AUS can be found at temperatures below the A1. Which of the following is not a reason?
a. diffusion rates in solids are slow
b. tempering is a time dependent process
c. the transformation to MAR is temperature dependent
d. some alloying elements have extensive solubilities in gamma-Fe
11. Carbide tools are made by
a. heating together a mixture of Scheelite and tungsten carbide, followed by pressing
b. pressing a mixture of Scheelite and tungsten carbide powders, followed by sintering
c. melting a mixture of cobalt and tungsten carbide powders, followed by solidification
d. pressing a mixture of cobalt metal and tungsten carbide powders, followed by sintering
e. pressing a mixture of tungsten metal and carbon powders, followed by sintering
12. Not a class of stainless steels
a. martensitic
b. bainitic
c. austenitic
d. ferritic
13. Mainly responsible for diffusion in solids
a. thermal vibrations and vacancies
b. vacancies, interstitial atoms, and dislocations
c. vacancies, dislocations, and grain boundaries
d. thermal vibrations and dislocations
e. melting
14. Proeutectoid phase formed upon slowly cooling a eutectoid steel (0.77 w/o C) from AUS
a. Pearlite
b. MAR
c. CM
d. FE
e. you've got to be kidding!
15. Proeutectoid phase formed upon cooling a 1060 steel
a. Pearlite
b. MAR
c. CM
d. FE
e. AUS
16. Equation which represents the temperature dependence of diffusion in solids
a. îG = îH - TîS
b. n/no = exp (-Q/RT)
c. n/no = exp (Q/RT)
d. D(T) = Do exp (-Q/RT)
e. D(T) = Do exp (Q/RT)
17. AUS --> MAR transformation
a. time dependent, temperature dependent
b. time dependent, temperature independent
c. time independent, temperature independent
d. time independent, temperature dependent
e. an equilibrium reaction
18. Phases present in a hypereutectoid steel slowly cooled to room temperature from the AUS phase:
a. FE, P
b. CM, P
c. FE, CM
d. MAR
e. MAR, P
19. Determines the maximum attainable hardness of MAR
a. carbon content
b. carbon content and alloy element content
c. dislocation density
d. extent of cold working
e. rate of quenching
20. For C diffusing in iron, the ratio, D(1000°C)/D(500°C), is roughly
a. 2
b. 10
c. 100
d. 1000
e. 10,000
21. For tool steels designations
a. H stands for hot oil quench
b. A stands for any kind of quench
c. S stands for high strength steel
d. O indicates oil lubrication required when used for machining
e. W grades have relatively low hardenability
22. Upon tempering a Cr-containing steel (the tempering time can vary), which is not true
a. AUS sometimes forms
b. Fe2.2C sometimes forms
c. Cr7C3 can form
d. FE can form
e. stress relief occurs
23. A hypoeutectoid steel is slowly cooled from a temperature of 1000°C. Which is correct
a. there is no AUS decomposition
b. the composition of the steel does not change
c. the composition of the AUS does not change
d. the composition of the FE does not change
e. no P is formed
24. The ratio of the maximum solubility of C in ccp Fe to the solubility of C in "bcc" iron is about
a. 0.01
b. 0.1
c. 1
d. 10
e. 100
25. The phase MAR does not show on our Fe-C diagram because
a. MAR contains more than 6.67 w/o C
b. MAR only comes from AUS
c. P forms much faster than MAR
d. MAR forms by a structural shear process
e. the Fe-C diagram is an equilibrium diagram