1. Why are steels used so extensively?
iron is allotropic
2. What is the composition of a 4140 steel?
0.40 carbon 0.77 Mn 0.98 Cr 0.21 Mo -
according to table in notes, page 6: 41xx are 0.50Cr-0.12Mo or 0.95Cr-0.20Mo
3. Suggest a reason why rivets made of a 2017 aluminum alloy are refrigerated until they are used.
2017 similar to 2024 (Al-4.4Cu) age hardening alloy
want rivets to be soft for heading operation - after heading want strength
to increase markedly
after solution treatment atabout 500 C, apparently have to refrigerate alloy
to keep it from ageing
so rivets stay soft until after heading
alloy ages at room temperature
4. Compare age hardening of aluminum alloys and the quenching and tempering of steel with respect to the processing steps. How do the mechanical properties change during each step for both types of alloys.
Q&T and Q&A have same type of temperature
changes - i.e., heat to relatively high temp., quench, reheat to low temp.
for Q&T, at high temp. mat'l is soft. after quench mat'l is glass brittle.
after tempering, incredibly tough
for Q&A, mat'l is soft at high temp, soft after quenching, strong after
ageing
5. What is the purpose of annealing an alloy?
to soften it
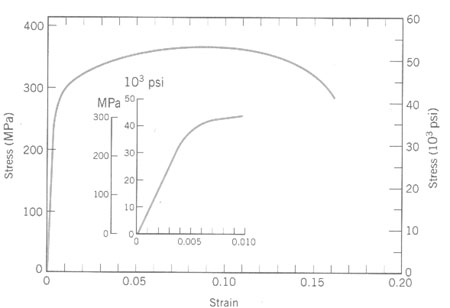
6. Determine yield strength, modulus of eleasticity, tensile strength, elongation, for data given below.

YS = 41 ksi at 0.2% offset, UTS = 53 ksi, elongation
= 15.7% (don't include elastic deform.)
modulus = slope of elastic portion = 18ksi/0.002 = 9000 ksi
7. Suppose a metal piece has a dislocation density of 104 /mm2. If all the dislocations in 1 cc of the material were somehow removed and linked end to end, how long would this chain be? If the materials was cold worked so that the dislocation density increased to 1010/mm2, how long would the chain be?
To estimate, assume dislocs in 1 cc cube are all
1 cm long and terminate on one face of cube
to give stated density. total no./sq. cm = density x 100. Answers are then
106 cm and 1012 cm -
6 mi and 6 million mi.!
8. Describe these strengthening mechanisms and the role that dislocations play in each:
grain size reduction
disloc pileups against grain boundaries.....more grain
boundary material in small grained mat'ls
strain hardening
work hardening - dislocation entanglements. deformation
produces so many new dislocations that they become entangled.
solid solution hardening
atom size differences give distorted slip planes -
disloc motion is retarded
precipitation or age hardening
dislocs hang up on tiny precipitate particles whose
slip planes (if there are any) are in the wrong orientation wrt to slip
planes in the matrix mat'l or crystal structure of precipitate is too complex
for well-defined slip planes