The Use of 5000 Series Aluminum Alloys in Automobile Body Panels
There are several factors which make the use of aluminum alloys in automobile applications necessary. CAFE (Corporate Average Fuel Economy) law sets minimum fuel efficiency levels at 27.5 mpg for passenger cars and 21.5 mpg for light trucks. These mileage regulations conflict with other government regulated mandates for safety features such as airbags, side-impact collision beams, and antilock braking systems and consumer demands for features such as power windows and door locks. All of these features add weight to the vehicle which reduces the fuel efficiency. To accommodate these conflicting demands, automobile manufacturers have been looking for low density materials to use in place of steel. Aluminum alloys appear to be the most promising of the lighter materials available.
Aluminum alloys are classified with a four digit system which is based upon the principal alloying element (Davis, 1993). Table 1 shows the principal alloying element along with the corresponding designation.
Table 1 Aluminum Alloy Classification System (by principal alloying element)
| Aluminum 99.00% | 1XXX |
| Copper | 2XXX |
| Manganese | 3XXX |
| Silicon | 4XXX |
| Magnesium | 5XXX |
| Magnesium & Silicon | 6XXX |
| Zinc | 7XXX |
| Other Elements | 8XXX |
| Unused Series | 9XXX |
For about 10 years, the automotive industry has been using aluminum in parts such as wheels and radiators. To further reduce vehicle weight, however, the possibility of using aluminum alloys in other applications is being explored. The use of 6XXX (aluminum-magnesium-silicon) and 2XXX (aluminum-copper) series alloys in automobile body panels has increased throughout much of Asia and Europe. The use of these alloys is limited because they require a high temperature baking cycle to acquire the needed strength for body panel applications. This strength is normally achieved by age hardening during the paint baking process. With the increased use of plastics in automobile paint, the corresponding baking is being done at much lower temperatures. The 6XXX and 2XXX series alloys must then be hardened with another age hardening cycle. To avoid this expensive process, other alloys or other less costly strengthening methods must be developed (Brown, Venie, and Woods, 1995).
The use of 5XXX series alloys (aluminum-magnesium) is one possible alternative. 5XXX series alloys are work hardenable and thus do not require the paint baking cycle to achieve the necessary strength for use in automobiles. These alloys have gained some limited use in interior, non-visible, body panels such as the inside of trunk lids. In the processing of 5XXX series aluminum alloys, however, stretcher-strain markings, similar to the Lüders bands seen in steels, develop (Phillips, Swain, and Eborall, 1952). These marks can be easily seen even after painting, so a method must be developed to prevent them before these alloys can be used on a larger scale.
Lüders lines are regions of deformed material that develop in areas
of high stress concentration (Reed-Hill and Abbaschian, 1994). If the material
is held in tension, these deformations appear as indented regions where
the material is strained at a higher rate than the surrounding material.
In compression, the deformations are localized regions in which the material
is raised with respect to the surrounding areas. In either case, the Lüders
lines look like ripples in the surface of the sample. In 5XXX series alloys,
these markings develop because Mg, which is concentrated at grain boundaries,
prevents the transfer of strain from grain to grain (Sanders, Baumann, and
Stumpf, 1989). ¶ There
are two types of Lüders lines: type A and type B. Type A Lüders
lines develop in a small, localized regions and rapidly spread throughout
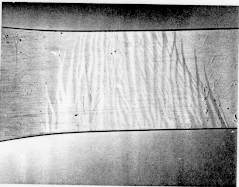
the material. Figure 1 shows typical type A Lüders lines which are
found in 5XXX series aluminum alloys. Figure 2 shows a typical stress-strain
curve for 5XXX series aluminum alloys. The large initial yield on this curve
(C) represents the rapid spread of the type A Lüders through the material.
The smaller, step-like yields in this figure (D and E) represent the development
of the type B Lüders bands. Type B markings form as many localized
regions within the material deform and produce small, individual yields
on the stress-strain curve.

Figure 1 Typical type A Lüders lines formed during tension in 5XXX series aluminum alloys (vertical wedges). The beginnings of type B lines can be seen on the right hand side of the photograph. (From Lloyd (1980).)needs size marker
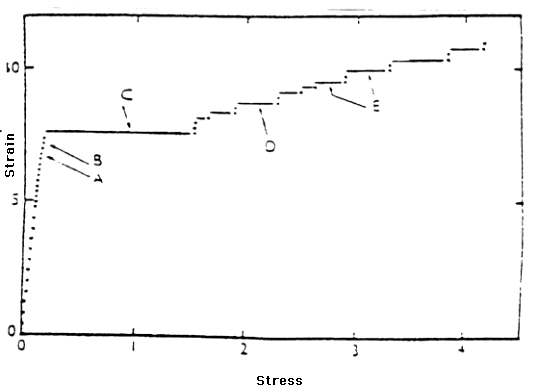
Figure 2 Stress/Strain curve for Al-3.5%Mg alloy as annealed. Grain size 0.018 mm. (From Phillips, Swain, and Eborall (1952).)
KEY
A. Small wedge at one extensometer pip.
B. Wedge spread a little.
C. Inclined sharp boundary ran down gauge length and one ran up to meet
it.
D. Ripple (type B marking) ran part way along gauge length, through slight
irregularities left from large yield
E. Several new ripples moved.
Several methods can be used to prevent the formation of type A Lüders lines. The introduction of dislocations in the alloy by either cold-rolling or straining the material past its yield point will effectively prevent type A markings from formingwhy??. The solute atoms (Mg) diffuse to these dislocations and pin them in place which prevents a large, type A yield. This process is known as strain aging.
Controlling the grain size is another method of preventing type A Lüders lines. By keeping the grain sizes larger than 0.05 mm, these markings can be virtually eliminated why??(Phillips, Swain, and Eborall, 1952). ¶None of these hasve any significant effect on the formation of type B markings. These markings can be prevented if the material is processed at low temperatures because the magnesium atoms can not gain enough energy to diffuse to the grain boundaries, and, thus, deformation occurs more smoothly. you are telling me that Mg atoms can move around easily at rm temp? would be nice to see the diffusion data!This method is rather costly and therefore not very practical for large scale uses. The development of an alternate method for removing type B Lüders lines is the last major hurdle to be overcome in order for the use of 5XXX series aluminum alloys to become practical.
To help understand how type B lines can be eliminated, a greater understanding of the mechanisms of Lüders band formation is needed. Type B Lüders lines are thought to be formed through the Cottrell mechanism (Phillips, Swain, and Eborall, 1952). In this mechanism, solute atoms (mostly Mg in 5XXX series alloys) diffuse to the grain boundaries. These atoms then slow the passage of mobile dislocations while surrounding atoms continue deforming plasticallyatoms deform plastically??. The stress builds until the dislocations finally break free and produce a localized deformation, or Lüders line. Figure 3 shows typical type B Lüders bands that are found in 5XXX series alloys. These bands form at 50° to the tensile axis. Figure 4 shows the configuration of the slip bands in an Al-0.3%Mg alloy before and after Lüders band formation. The dislocation structures of the framed regions M1 and M2 are shown in Figure 5. The picture on the right (M2) shows how the dislocations are stopped as they come up against the clusters of solute atoms (the white circular regions). The left picture (M1) shows how the dislocations have spread throughout the material after Lüders band formation.
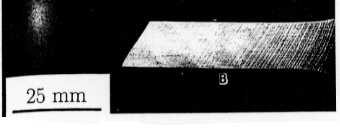
Figure 3 Typical type B Lüders lines formed during tension in 5XXX series aluminum alloys. Type B deformations form at about 50 to the tensile axis. (From Robinson and Shaw (1994).)

Figure 4 The configuration of the slip bands in an Al-0.3%Mg alloy before and after Lüders band formation. The dislocation structures of the framed regions M1 and M2 are shown below in Figure 5. (From Tabata, Fujita, and Ueda (1980).)
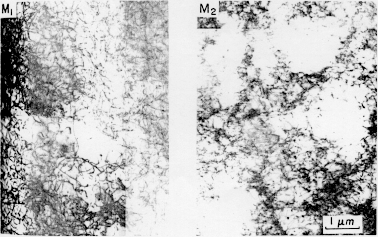
Figure 5 Dislocation structure for an Al- 0.3%Mg alloy. The picture on the right (M2) is before the formation of Lüders lines. The picture on the left (M1) is after the Lüders lines have formed. (From Tabata, Fujita, and Ueda (1980).)
Lüders line formation is dependent upon the temperature at which the material is deformed. In Al-Mg alloys, Lüdering occurs at temperatures between 200 and 350 K (Lloyd, 1980). At temperatures lower than 200 K the solute atoms cannot diffuse to the grain boundaries as easily, so the dislocations can flow more easily. Figure 6 demonstrates the temperature dependence of Lüders line formation by showing stress vs. strain curves for several temperatures. As the temperature is lowered to -76°C, Lüders line formation is prevented. At temperatures above 350 K, due to the higher ductility of these alloys at elevated temperatures, there is more uniform deformation and Lüders lines do not form.
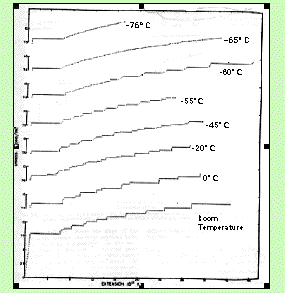
Figure 6 Stress vs. Strain curves at several temperatures for a 5XXX series aluminum alloy. At -76°C and below, type B Lüders band formation is prevented. (From Phillips, Swain, and Eborall (1952).)
The formation of Lüders lines is also dependent on grain size. Fujita and Tabata (1977) state that a small amount of deformation occurs among several grains rather than a large deformation in only one. In materials with smaller grain sizes, there are more grains which can cooperate to produce these deformations and the resulting LÅders bands. With larger grain sizes, there is less interaction among grains and, thus, there are fewer Lüders lines than in materials with smaller grain sizes. Figure 7 shows stress vs. strain curves for several different grain sizes. The steps on the curves become less jagged as the grain size is increased. Above 0.05 mm (not shown), Lüders lines are not formed.
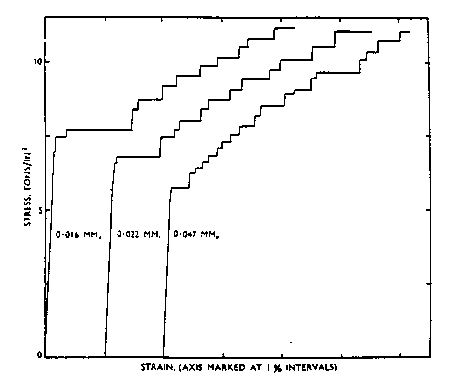
Figure 7 Stress-strain curves for several different grain sizes of a 5XXX series aluminum alloy. These graphs show how the curves have a more smooth appearance with increasing grain size. At grain sizes above 0.05 mm, Lüders lines are eliminated. (From Phillips, Swain, and Eborall (1952).)
Tabata, Fujita, and Ueda (1980) studied the effect of grain orientation on Lüders band formation in single crystal Al-0.3%Mg alloys. The different grain orientations were tested by using three different tensile axes. One axis initiated slip in a single plane, a second in two planes, and the third in six different planes. The double slip system was the only one why?? to form Lüders bands. The bands formed because the solute atoms effectively pinned down the mobile dislocations of the conjugate slip plane. These dislocations in the conjugate slip plane break free as the stress concentration increases and form Lüders lines.
In some aluminum alloys, type B Lüders can be eliminated by cold rolling the material. In Al-Mn-Mg alloys, a 20% cold reduction entirely suppressed the formation of Lüders. A 25% reduction eliminated most deformations and a 50% reduction removed them completely in an Al-5%Zn-1%Mg alloy (Robinson and Shaw, 1994). The cold work is believed to have created enough dislocations in the materials to overcome the normal effects of the Cottrell mechanism. This is the same process which prevents the formation of type A Lüders lines. In binary Al-Mg alloys, however, reductions of up to 75% have failed to produce the same type of results. In this case, the cold work is believed to enhance the solute diffusivity by creating vacancies.
REFERENCES
BROWN, K. R., VENIE, M. S., and WOODS, R. A., 1995, JOM, 47(7), 20
DAVIS, J. R., ed., 1993, Aluminum and Aluminum Alloys, ASM Specialty Handbook
(Materials Park: ASM International), p. 18
FUJITA, H. and TABATA, T., Acta Metall., 25, 181 (1987)
LLOYD, D. J., Metall. Trans. A, 11, 1287 (1980)
REED-HILL, E. and ABBASCHIAN, R., 1994, Physical Metallurgy Principles (Boston:
PWS Publishing), p. 285
ROBINSON, J. M. and SHAW, M. P., Int. Mater. R., 39, 113 (1994)
SANDERS, R. E., Jr., BAUMANN, S. F., and STUMPF, H. C., 1989, in Aluminum
Alloys -Contemporary Research and Applications (New York: Academic Press), A. K.
Vasedovan and R. D. Doherty, eds., p. 96
SWAIN, V. A., SWAIN, A. J., and EBORALL, R., J. Inst. Met., 81, 625 (1952)
TABATA, T., FUJITA, H., and UEDA, N., Mater. Sci. E., 44, 81 (1980)