
The Mechanisms of Lüders Band Formation
An effective method for preventing type B Lüders lines must be developed before 5XXX series aluminum alloys can be used in automobile body panels. To help understand how these lines can be eliminated, a greater understanding of the mechanisms of Lüders band formation is needed. Type B Lüders lines are thought to be formed through the Cottrell mechanism (Phillips, Swain, and Eborall, 1952). In this mechanism, solute atoms (mostly Mg in 5XXX series alloys) diffuse to the grain boundariesstill in solution??. These atoms then slow the passage of mobile dislocationswhile surrounding regions continue deforming plastically. Would be nice to have a drawing which shows geometrically how the surface distortsThe stress builds until the dislocations finally break free and produce a localized deformation, or Lüders line. Figure 1 shows typical types A and B Lüders found in 5XXX series aluminum alloys (Robinson and Shaw, 1994). Figure 2 shows the dislocation structure before and after Lüders formation in an Al-0.3%Mg alloy (Tabata, Fujita, and Ueda, 1980). In this figure, the picture on the right shows how the dislocations are stopped as they come up against the clusters of solute atoms (the white circular regions). The left picture shows how the dislocations have spread throughout the material after Lüders band formationI'd probably interchange the pics.

Figure 1 Typical type Acan't see and type B Lüders formed during tension in 5XXX series aluminum alloys. The type B deformations form at about 50 to the tensile axis.
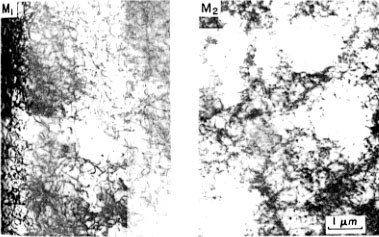
Figure 2 Dislocation structure for an Al- 0.3%Mg alloy. The picture on the right is before the formation of Lüders lines. The picture on the left is after the Lüders lines have formedneeds micron marker.
Lüder line formation is dependent upon the temperture at
which the material is formed. In Al-Mg alloys,
it has been shown that Lüdering occurs at temperatures between
200 and 350 K (Lloyd, 1980). At temperatures lower than 200 K the
solute atoms cannot diffuse to the grain boundaries
as easily, so the dislocations can flow more
easilyer. At temperatures above 350K,
due to the higher ductility of the alloy at elevated temperatures, there
is more uniform deformation and Lüders lines
do not form. The formation of Lüders lines is also dependent
on grain size. Fujita and Tabata (1977) state
that a small amount of deformation occurs among several grains rather than
a large deformation in only one (1977).need
to know how, why this works....again, a diagram which shows the geometry
would be nice In materials with smaller grain sizes, there are more
grains which can cooperate to produce these deformations and the resulting
LÅders bands. With larger grain sizes there is less interaction
among grains and, thus, there are less Lüders lines than in materials
with smaller grain sizes.
Tabata, Fujita, and Ueda (1980) studied
the effect of grain orientation on Lüders band formation in single
crystal Al-0.3%Mg alloys (1980). The different grain orientations
were tested by using three different tensile axes. One axis initiated slip
in a single plane, a second in two planes, and the third in six different
planes. The double slip system was the only one to form Lüders bands.Aha!!! The bands formed because the solute atoms
effectively pinned down the mobile dislocations of the conjugate slip plane.
These dislocations in the conjugate slip plane break free as the stress
concentration increases and form Lüders lines.
In some aluminum alloys, type B Lüders can be eliminated by cold
rolling the material. In Al-Mn-Mg alloys, a 20% cold reduction of area entirely
suppressed the formation of Lüders. A 25% reduction eliminated most
deformations and a 50% reduction removed them completely in an Al-5%Zn-1%Mg
alloy (Robinson and Shaw, 1994). The cold work is believed to have created
enough dislocations in the materials to overcome the normal effects of the
Cottrell mechanism. In binary Al-Mg alloys, however, reductions of up to
75% have failed to produce the same type of results. In this case, the cold
work is believed to enhance the solute diffusivity by creating vacancies.
why the difference?? The higher Mg concentration
at the grain boundaries enables Lüders to form by the Cottrell mechanism.
REFERENCES
FUJITA, H. and TABATA, T., Acta Metall., 25, 181 (1987)
LLOYD, D. J., Metall. Trans. A, 11, 1287 (1980)
ROBINSON, J. M. and SHAW, M. P., Int. Mater. R., 39, 113 (1994)
SWAIN, V. A., SWAIN, A. J., and EBORALL, R., J. Inst. Met., 81, 625 (1952)
TABATA, T., FUJITA, H., and UEDA, N., Mater. Sci. E., 44, 81 (1980)