The Use of 5000 Series Aluminum Alloys in Automobile
Body Panels
writer's name
writer's email address
Note: red is instructor's suggested addition or comment; stuck-through red is suggested deletion.
There are several factors which are combining
tomake the use of aluminum alloys in automobile applications
more necessary than?. The United States
Congress has passed CAFE (Corporate Average Fuel Economy) law
which sets minimum fuel efficiency levels. These mandates
currently require an average of at least 27.5 mpg for passenger cars and
21.5 mpg for light trucks. These mileage regulations conflict with other
government regulated mandates for safety features such as airbags, side-impact
collision beams, and antilock braking systems. Consumer demands for features
such as power windows and door locks also extra space
here reduce fuel efficiencydo the demands reduce
the fuel efficiency?. All of these extra features add significant
weightwhat kind of weight is significant weight?
which is detrimental to fuel efficiency. To accommodate these conflicting
demands, automobile manufacturers have been looking for low
densitylighter materials to use in place of steel. Aluminum
alloys appear to be the most promising of the lighter materials available.
Wordy....collapse ¶ to probably two sentences
Aluminum alloys are classified with a four digit system which is based upon the principle alloying element (1). Table 1 shows the principal alloying element along with the corresponding designation.
Table 1 Aluminum Alloy Classification System (by principal alloying element)
Aluminum > 99.00% 1XXX
Copper 2XXX
Manganese 3XXX
Silicon 4XXX
Magnesium 5XXX
Magnesium & Silicon 6XXX
Zinc 7XXX
Other Elements 8XXX
Unused Series 9XXX
For about 10 years, the automotive industry has been using aluminum in parts like wheels and radiators. To further reduce vehicle weight however, the possibility of using aluminum alloys in other applications is being explored. The use of 6XXX (aluminum-magnesium-silicon) and 2XXX (aluminum-copper) series alloys in automobile body panels has increased throughout much of Asia and Europe. The use of these alloys is limited because they require a high temperature baking cycle to acquire the needed strength for body panel applications. This strength is normally achieved by age hardening during the paint baking process. With the increased use of plastics in automobile paint, the corresponding baking is being done at much lower temperatures. The 6XXX and 2XXX series alloys must then be hardened with another age hardening cycle. To avoid this expensive process, other alloys or other less costly strengthening methods must be developed (2).
The use of 5XXX series alloys (aluminum-magnesium) is one possible alternative.
5XXX series alloys are work hardenable and thus do not require the paint
baking cycle to achieve the necessary strength for use in automobiles. These
alloys have gained some limited use in interior, non-visible, body panels
such as the inside of trunk lids. There is a problem
which must be overcome before the 5XXX series alloys can be used on exterior
body panels (2). In processing 5XXX series aluminum alloys, however, stretcher-strain markings, similar to the
Lüders bands seen in steels, develop (3). These marks can be easily
seen even after painting, and so a method
must be developed to prevent themthese marks
before these alloys can be used on a larger scale.
Lüders lines are regions of deformed material that develop in areas
of high stress concentration (4). If the material is held in tension, these
deformations appear as indented regions, where the material is strained
at a higher rate than the surrounding material. In compression, the deformations
aretake the form of localized regions
in which the material is raised with respect to the surrounding areas. In
either case, the Lüders lines look like ripples in the surface of the
sample. In 5XXX series alloys, these markings develop due to concentrations
of magnesiumMg? at grain boundaries which preventverb says subject is concentrations....but isn't it the
Mg (?) that prevents--> the transfer of strain from grain to grain
(5). There are two types of Lüders lines which
form: tType A and tType
B. Type A Lüders lines develop in a small,
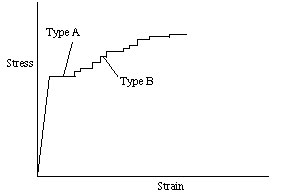
localized regions and rapidly spread throughout the material. Figure 1 showscontains a typical
stress-strain curve forthat is typical of
5XXX series aluminum alloys (3). The large initial yields on these curves
represent extra space here the rapid spread
of the Type A Lüders lines through the
material. The smaller, step-like yields in this figure represent the development
of the Type B Lüders lines or bands. Type
B markings form as many....many what?;,
localized regions within the material deform and produce small, individual
yields on the stress-strain curve.

Figure 1 Typical Stress/Strain curve for 5XXX series aluminum alloys
,There are sSeveral methods which can be used to prevent the formation of
Type A Lüders..... The introduction of
dislocations in the alloy by either rolling or straining the material past
its yield point will effectively prevent the
Type A markings from forming. Controlling the grain size of
the material is another method of preventing Type A Lüders.
By keeping the grain sizes larger than 0.05 mm, these markings can be virtually
eliminated why?(3). Type
B Lüders are much more difficult to prevent, however. None
of these methods used
to prevent Type A hasve any
significant effect on the formation of Type B markings. These markings can
be prevented if the material is processed at low temperatures because the
magnesium atoms can not gain enough energy to diffuse to the grain boundaries,
and thus, deformation occurs more smoothly. This method is rather
costly and therefore not very practical for large scale uses. The development
of an alternate method for removing Type B Lüders is the last major
hurdle to be overcome in order for the use of 5XXX series aluminum alloys
to become practical.
REFERENCES
1. J. R. Davis, ed., Aluminum and Aluminum Alloys, ASM Specialty Handbook, ASM
International, Materials Park, 1993, p. 18
2. K. R. Brown, M. S. Venie, and R. A. Woods, JOM, v.47(7), 1995, p.20
3. V. A. Phillips, A. J. Swain, and R, Eborall, J. Inst. Metals, v.81, 1952-1953, p. 626
4. R. E. Reed-Hill, and R. Abbaschian, Physical Metallurgy Principles, PWS Publishing,
Boston, 1994, p. 285
5. R. E. Sanders, Jr, S. F. Baumann, and H. C. Stumpf, in Aluminum Alloys -
Contemporary Research and Applications, A. K. Vasedovan and R. D. Doherty, eds.,
Academic Press, New York, 1989, p. 96