Homework 5
X-ray Fluorescence
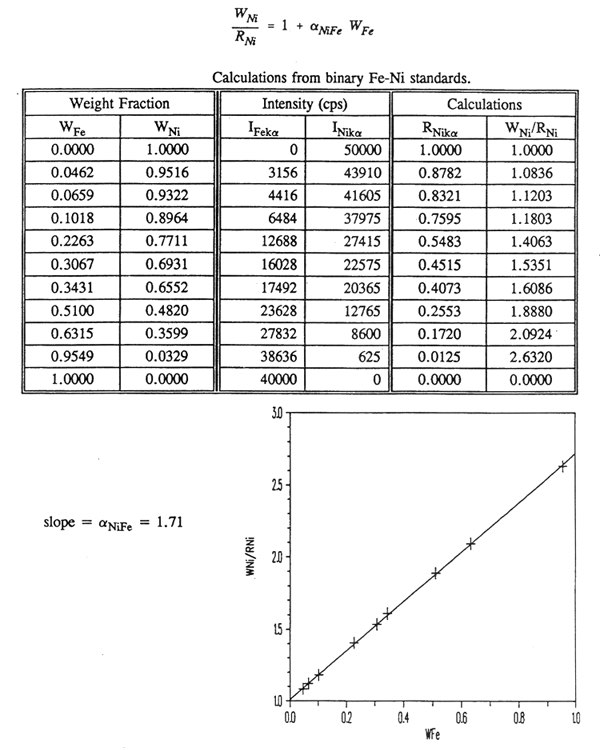
a. Standard binary mixtures of Fe and Ni were made up and the intensities of the x-ray spectra lines were measured as given in the table below. Write the equation for W/R for this type of binary mixture and determine alpha(NiFe).

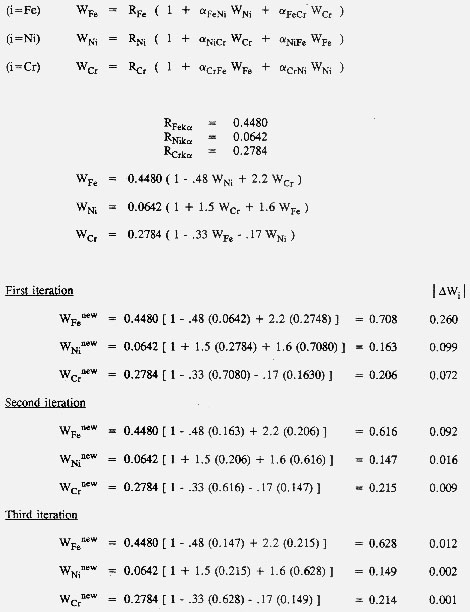
b. An analysis of an Fe-Ni-Cr mixture was made, and the resulting R values were
RFeKalpha = 0.4480
RNiKalpha = 0.0642
RCrKalpha = 0.2784
Write the three simultaneous equations for Wi.
see below
c. The influence coefficients for these equations were determined from measurements on binary standards using the technique in part a above. The results were
alpha(FeNi) = -0.48 alpha(FeCr) = 2.2 alpha(NiCr) = 1.5
alpha(CrFe) = -0.33 alpha(CrNi) = -0.17
Determine the Wis by an iterative technique. Choose appropriate values for the initial values of the Wis on the right side of the equations above. (Hint: what if all the influence coefficients were zero?) As each new value of the Wis is calculated, insert it into the subsequent equations. Do three iterations; i.e., successively calculate three sets of Wis. Plot the three Wis as a function of iteration number to judge whether the three iterations are sufficient to determine the Wis to within 2%.