X-ray tubes and tube spectra
X-rays were discovered by W. Rontgen in Würzburg, Germany, in 1895.
The modern x-ray tube operates in the same manner as the one that Rontgen
used. Electrons are "boiled" off of a heated thoriated tungsten
filament and accelerated toward a target or anode where the x-rays are produced.
The Coolidge-type x-ray tube is sealed and operates under a relatively high
vacuum. The focusing cup, held at a negative potential, helps to direct
the electrons toward the anode. To adjust the x-ray beam intensity, the
tube current and/or the tube voltage can be changed, but the IV power is
limited because of the extensive heat development in the target material.
About 99% of the energy input is converted to heat. As a result, the anode
must be water-cooled. The energy of the electrons as they hit the target
is given by eV = hc/ ![]() because of their dual wave-particle nature.
because of their dual wave-particle nature.
![]() = hc/eV = 12.4/V (V in kV and
= hc/eV = 12.4/V (V in kV and ![]() in Å).
in Å).
White or continuous radiation:
White or continuous radiation is produced by the deceleration of electrons
as they hit the target. (Decelerating charged particles give up their energy
in the form of radiation.) This radiation is also called Bremstrahlung or
"braking" radiation. A spectrum of continuous radiation is obtained
since the electrons can exchange any or all of their energy upon interaction
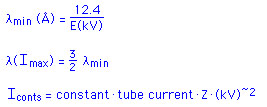
with the atoms in the target. The shortest wavelength (![]() SWL)
in the spectrum arises from an exchange of all the energy of an electron,
which is a function of the applied voltage. The total intensity of the continuous
radiation is Iconts = AiZV~2 , where A is a constant,
i is the tube current, and Z is the atomic number of the target. There is
only one common technique that specifically employs this type of radiation
- the Laue technique for single crystals; an x-ray tube with a target of
high atomic number is generally used for Laue photographs.
SWL)
in the spectrum arises from an exchange of all the energy of an electron,
which is a function of the applied voltage. The total intensity of the continuous
radiation is Iconts = AiZV~2 , where A is a constant,
i is the tube current, and Z is the atomic number of the target. There is
only one common technique that specifically employs this type of radiation
- the Laue technique for single crystals; an x-ray tube with a target of
high atomic number is generally used for Laue photographs.
Characteristic radiation:
Characteristic radiation is produced through the ejection, upon collision, of an electron from one of the energy levels of the atoms of the target material; the x-rays are produced when the empty level is filled again by a transition of an electron from a higher level to the empty level. (Decelerating charged particles give up their energy in the form of radiation.) This type of radiation is characteristic of the target material. Different target materials give off characteristic x-rays of different wavelengths according to Moseley's law
![]()
where C and ![]() are constants. Transitions that terminate on the K
level (and in a few cases the L levels) give rise to x-rays with wavelengths
in the useful range of about 0.5-5 Å. There are two L
are constants. Transitions that terminate on the K
level (and in a few cases the L levels) give rise to x-rays with wavelengths
in the useful range of about 0.5-5 Å. There are two L![]() K transitions
(which give K
K transitions
(which give K![]() 1 and K
1 and K![]() 2 x-rays) and one M
2 x-rays) and one M![]() K transition (which
gives K
K transition (which
gives K![]() x-rays) allowed which are important.
x-rays) allowed which are important.
![]()
The wavelengths of the K![]() 1 and K
1 and K![]() 2 x-rays are
so close together that they are frequently not very well resolved in the
peaks in x-ray diffraction patterns, and therefore, to interpret such measurements,
a weighted average of the wavelengths is used:
2 x-rays are
so close together that they are frequently not very well resolved in the
peaks in x-ray diffraction patterns, and therefore, to interpret such measurements,
a weighted average of the wavelengths is used:
![]()
A minimum voltage (Vcrit = 12.4/ ![]() K, where
K, where ![]() K
is the wavelength of the "K edge") on the x-ray tube is required
in order to obtain emission of the characteristic radiation since the incoming
electrons must have an energy great enough to eject the electrons from the
atoms of the target material. For example, to calculate the minimum voltage
at which a iron target x-ray tube must be operated in order to produce the
characteristic x-rays for iron, we look up the K edge for iron and calculate:
K
is the wavelength of the "K edge") on the x-ray tube is required
in order to obtain emission of the characteristic radiation since the incoming
electrons must have an energy great enough to eject the electrons from the
atoms of the target material. For example, to calculate the minimum voltage
at which a iron target x-ray tube must be operated in order to produce the
characteristic x-rays for iron, we look up the K edge for iron and calculate:
12.4 Å/kV
1.743 Å = 7.11 kV.
The intensity of the characteristic radiation is given by Ichar = Ai(V-Vcrit)~1.5. This equation shows that the intensity of the characteristic radiation at V < Vcrit is zero, and that it increases as V increases above Vcrit.
The characteristic and continuous spectra superimpose upon one another, and the resulting wavelength distribution is what the sample usually sees.
SS#_________________________________
Grade_____________/10
MatSE 430 - Problem 1 - Due 8/31/00
X-ray Spectra
The problem below will help you fix on the above concepts, and help you understand the setup and the operating conditions for your x-ray generators.
a. Sketch the curve of intensity versus wavelength for a Cu target X-ray tube operated at 5 milliamperes and 5, 10, and 25 kilovolts.
To sketch the continuous radiation component, note the following.
For the characteristic lines of the spectra, refer to the appropriate appendix in Jenkins & Snyder or Cullity. Also:
![]()
b. How would the curve for 5 kV change if the X-ray tube current is raised to 10 mA?
Problem 2
Braggs' law calculations
a. Find 2![]() hkl for dhkl = 2.00, 1.50, and 0.707 Å for
CuK
hkl for dhkl = 2.00, 1.50, and 0.707 Å for
CuK![]() 1 radiation.
1 radiation.
b. For CuK![]() 1 radiation, suppose that the smallest 2
1 radiation, suppose that the smallest 2![]() at which a measurement
can be made is 10°. What, then, is the largest d that can be measured?
If 2
at which a measurement
can be made is 10°. What, then, is the largest d that can be measured?
If 2![]() max = 160°, what is the smallest d that can be measured?
max = 160°, what is the smallest d that can be measured?
c. For a cubic crystal, the interplanar spacings can be calculated from the lattice parameter (the length of the cubic cell edge), a, and the Miller indices (hkl) of the planes according to the equation:
![]()
Combine this equation with Braggs' law:
![]()
and determine the 2![]() angles at which the first three reflections for nickel
metal occur for CuKa1 radiation. For this F cubic metal, the
first three reflections are (111), (200), and (220), and a = 3.5239 Å.
angles at which the first three reflections for nickel
metal occur for CuKa1 radiation. For this F cubic metal, the
first three reflections are (111), (200), and (220), and a = 3.5239 Å.
Problem 3
More Braggs' law calculations
Calculate and list in order of increasing angle the (hkl) and 2![]() values
for the first three reflections for the following, using
values
for the first three reflections for the following, using
CuK![]() radiation.
radiation.
a. primitive cubic, a = 3.00 Å
The next two calculations involve a tetragonal lattice. The unit cell is not a cube; you can picture a tetragonal unit cell as a cube that has been either squashed or extended along one lattice translation direction. As a result, one of the lattice translation distances (c) is no longer equal to the other two (|a| = |b|). The equation which relates h, k, l, a, and c to dhkl is:
![]()
b. primitive tetragonal, a = 2.00, c = 3.00 Å
c. primitive tetragonal, a = 3.00, c = 2.00 Å