Modeling Carbon Isotopes
Natural Variations in Carbon Isotope Ratios
Changes in Isotopic Ratios of Reservoirs
A Simple Atmospheric d13C
Model
1. Preliminary Effects of Fossil Fuel Emissions
3. Adding Outflows -- Exploring the Problem of the Missing Sink
Exploring the Dynamics of the Cretaceous-Tertiary
Crisis
Background on the K/T Mass Extinction
Iridium and the Impact Hypothesis
Modeling the K/T Impact Crisis
2. Varying the Magnitude and Duration of the Kill
3. Effect of Large-Scale Global Fires
Introduction
In the introduction to this chapter, we saw
that there was some very useful information contained in the changing ratio of 13C
to 12C over time. In this section, we will explore the world of
carbon isotopes in greater detail as we develop and experiment with a variety
of models. Some of these models will enable us to better understand the
relationship between burning fossil fuels and the atmospheric 13C /12C
ratio, including how different possibilities for the "missing sink"
affect the history of in the atmospheric 13C /12C ratio.
A more complex model will be used as a means for understanding what happened to
the carbon cycle in the oceans during the crisis at the Cretaceous-Tertiary
boundary, 65 million years ago, when Earth was hit by a very large asteroid,
producing some very dramatic, catastrophic changes to the climate.
Isotopes of Carbon
As mentioned earlier, there are a variety of
naturally-occurring isotopes of carbon. These isotopes are characterized by
differing atomic weights resulting from varying numbers of neutrons in the
atomic nuclei. The relative abundances of these isotopes are given below:
12C 98.89%
13C 1.11%
14C 1E-10%
12C and 13C are both
stable isotopes, meaning that unlike their radioactive cohort, 14C, they do not undergo radioactive decay. 14C is constantly being produced in the atmosphere as
nitrogen atoms are bombarded by high-energy solar radiation, but it is also
being destroyed (converted to 12C ) as it undergoes radioactive
decay. 14C has also been
produced during atmospheric nuclear explosions, most of which occurred in the
early 1960s. The production of 14C
is also dependent on things like the variations in solar output and the
strength of the Earth's magnetic field, which acts to shield out the radiation
that produces 14C.
Isotopic ratios are typically represented as
deviations relative to a standard ratio that occurs in some
"standard" material. The format for reporting these ratios is shown
in the equation below:

This is simply the fractional difference
between a sample and the standard, only here, it is actually a per mil (‰) difference
rather than a percent (%) difference since the values tend to be so small.
Looking at the above equation, we can see that if a sample has a greater
proportion of the heavier isotope of carbon, 13C, then it will have
a positive d 13C value and if it is depleted in the
heavier isotope, then it will have a negative d 13C
value. The standard was originally the carbon contained in calcite from fossils
called belemnites from a particular limestone called the Pee Dee Formation
(abbreviated as PDB).
Fractionation of Isotopes
Earlier in this chapter, we mentioned that
the carbon released from burning fossil fuels has a lower d13C (-25 to -20‰) than the atmosphere (-8‰). Why is this so?
A partial explanation is that fossil fuels are derived from a combination of
terrestrial and marine organic material, and if we look at the carbon isotope
values in those material today, we see that they have d 13C values of around -25‰ to -20‰. But then the question
becomes: Why are the d 13C values of modern organic matter so
different from the atmosphere? This brings us to the underlying explanation --
the process of photosynthesis favors the lighter form of carbon.
Photosynthesis, along with a few other processes, lead to variations in carbon
isotope ratios. These processes are sometimes called isotope fractionation
processes, which turn out to be very useful in helping us to decipher the past
behavior of the carbon cycle.
Natural Variations in Carbon Isotope
Ratios
The various fractionation processes lead to
the natural variations shown in Figure 7.17.
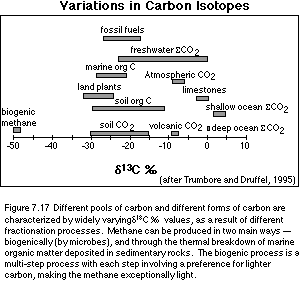
There are a few features in this figure that are worth discussing. One is that land plants, soil organic matter, soil CO2, marine organic matter, and fossil fuels all tend to hover in the -20 to -30‰ range. This reflects the fact that photosynthesis -- on land or in the sea -- always takes more of the lighter carbon from the mix of available CO2. This means that the carbon fixed by plants will always have a d13C value that is less than that of the source CO2. This shift is generally in the range of -20 to -30‰, depending on a variety of environmental factors, and the details of the process of photosynthesis. This fractionation or discrimination involved with photosynthesis explains several other features of Figure 7.17. The shallow oceans are more positive than the deeper oceans because planktonic organisms take the lighter carbon out of the shallow water to make everything but their shells. Removing the light carbon will naturally leave the sea water depleted in the lighter carbon, so the sea water itself becomes more positive. When these organisms die, they sink to the deeper parts of the oceans, where their organic remains are largely decomposed, returning the carbon to the water, causing the deeper waters to be less positive than the surface waters. The difference between the surface d 13C and the deep ocean d 13C is a measure of the efficiency of the biologic pump -- this difference can be measured by studying the shells of surface-dwellers and bottom-dwellers that are preserved in deep-sea sediments. Limestones will tend to sample and preserve the carbon isotopic signature of the ocean water where the majority of the organisms whose shells make up the limestone were living, with perhaps a small effect from the incorporation of a bit of organic material. The extreme outlier in above figure is methane, which is extremely negative as a result of being the end-product of several steps carried out by microbes wherein each step involves a preference for the lighter form of carbon.
Changes in Isotopic Ratios of Reservoirs
To make a model that monitors the isotopic
ratio of carbon in various reservoirs, we need to develop some equations, but
first, it will help to solidify a few very simple ideas by considering a couple
of examples -- simple thought experiments.
Imagine that we have a reservoir of carbon
that has an initial 13C /12C ratio and then we add new
carbon with the same ratio as the carbon that was already in the reservoir. The
total amount of carbon will have increased, but the ratio of carbon isotopes
will remain the same. Next, imagine what happens if we add carbon with a
smaller ratio of 13C /12C -- the amount of carbon once
again increases, but the 13C /12C ratio of reservoir will
have decreased. How about if we remove carbon from the reservoir, and we
discriminate in this removal such that the carbon taken away has a smaller 13C
/12C ratio than the overall reservoir? In this case, the amount of
carbon in the reservoir will obviously decrease, but the 13C /12C
ratio in the reservoir will increase.
What we need for the purposes of a model is a
general equation that will calculate the changes in the isotopic values for
reservoirs as a function of the amount of carbon added or subtracted and the
ratio of that carbon relative to the carbon in reservoir. We start by
considering a reservoir called M that has an inflow Fi and an outflow Fo. The rate of change of the reservoir is just:
![]()
If the inflow and outflow transfer carbon
with specific d13C values, di and do, and if we know the
starting d13C for the reservoir, dM, we can write a similar equation to the above that
incorporates these isotopic ratios:
![]()
But what we're really after is an equation
that tells us how dM
changes with time, so we need to deconstruct the
above equation (2). We apply the product rule of calculus to the above, giving:
![]() ,
,
and then we rearrange things in (3) to get:
![]() .
.
Then we substitute (1) into (4) to get the
following:
![]()
which can be simplified by rearranging terms
to give us our final equation:
![]()
This final equation then is essentially what
gets entered as the equation for a flow that feeds in and out of a reservoir
that is the isotopic ratio of the carbon contained in another reservoir. This
is a rather unusual kind of quantity to keep track of in a reservoir -- not as
intuitive as the mass of carbon in the atmosphere for instance.
Construction of a Simple Atmospheric d13C Model
Now it is time to apply what we learned in
the last section in the form of a very simple model that represents a small
portion of the carbon cycle. We will model only the atmospheric reservoir and
only one flow into the reservoir -- the carbon emitted from burning fossil
fuels. Our goal here is to represent the historical fossil fuel emissions and
their effect on the atmospheric d 13C
under some very simple assumptions. We assume that the atmosphere started out
with a d13C of -6, which is known from studying
tree rings and also from ice core measurements. The d 13C of fossil fuels varies (see above figure) with an
average of around -22‰, and the history of emissions is reasonably well known
-- it is included here as a graphical function of time, beginning in 1860 and
ending in 1990. The structure of our model is shown in Figure 7.18. Note that
the flow connected to the del 13C reservoir is a bi-flow rather than a uniflow
— this allows for an increase or a decrease in the reservoir quantity, while a
uniflow would only allow for an increase or a decrease (but not both).
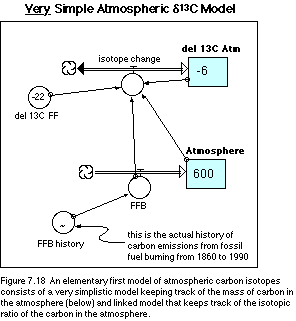
The equations that lie beneath this model structure are listed below. Note that there is a rather lengthy listing for the FFB (fossil fuel burning) history -- this is the most detailed resolution available and it means that we have to adjust the beginning and ending times of our simulations (in the Time Specs dialog box) to go from 1860 to 1990. Because of the large number of data points here, I suggest that you load this model, which includes the pre-made graph for FFB history, from the disk that accompanies this book.
Equations for Very Simple Atmospheric d 13C Model
Reservoirs:
INIT Atmosphere = 600 {Gt C}
INIT del_13C_Atm = -6 {pre-industrial del13C}
Flows:
FFB = FFB_history {FFB stands for fossil fuel burning}
isotope_change = (FFB*(del_13C__FF-del_13C_Atm))/Atmosphere
Converters:
del_13C__FF = -22 {del13C of fossil fuels}
FFB_history = GRAPH(time)
(1860, 0.0933), (1861, 0.0987), (1862, 0.0984), (1863, 0.106), (1864, 0.115), (1865, 0.122), (1866, 0.129), (1867, 0.138), (1868, 0.137), (1869, 0.142), (1870, 0.145), (1871, 0.162), (1872, 0.176), (1873, 0.188), (1874, 0.184), (1875, 0.189), (1876, 0.192), (1877, 0.196), (1878, 0.197), (1879, 0.208), (1880, 0.227), (1881, 0.244), (1882, 0.263), (1883, 0.28), (1884, 0.282), (1885, 0.276), (1886, 0.279), (1887, 0.298), (1888, 0.322), (1889, 0.329), (1890, 0.35), (1891, 0.365), (1892, 0.369), (1893, 0.362), (1894, 0.377), (1895, 0.399), (1896, 0.412), (1897, 0.431), (1898, 0.455), (1899, 0.497), (1900, 0.525), (1901, 0.54), (1902, 0.553), (1903, 0.606), (1904, 0.613), (1905, 0.647), (1906, 0.696), (1907, 0.771), (1908, 0.737), (1909, 0.769), (1910, 0.805), (1911, 0.822), (1912, 0.866), (1913, 0.929), (1914, 0.838), (1915, 0.831), (1916, 0.895), (1917, 0.945), (1918, 0.932), (1919, 0.829), (1920, 0.959), (1921, 0.828), (1922, 0.891), (1923, 1.01), (1924, 0.999), (1925, 1.01), (1926, 1.01), (1927, 1.10), (1928, 1.09), (1929, 1.17), (1930, 1.08), (1931, 0.968), (1932, 0.874), (1933, 0.919), (1934, 0.997), (1935, 1.03), (1936, 1.15), (1937, 1.23), (1938, 1.16), (1939, 1.23), (1940, 1.30), (1941, 1.34), (1942, 1.33), (1943, 1.36), (1944, 1.35), (1945, 1.20), (1946, 1.27), (1947, 1.42), (1948, 1.52), (1949, 1.49), (1950, 1.64), (1951, 1.77), (1952, 1.80), (1953, 1.85), (1954, 1.87), (1955, 2.05), (1956, 2.19), (1957, 2.28), (1958, 2.34), (1959, 2.47), (1960, 2.59), (1961, 2.60), (1962, 2.71), (1963, 2.85), (1964, 3.02), (1965, 3.15), (1966, 3.31), (1967, 3.42), (1968, 3.60), (1969, 3.81), (1970, 4.08), (1971, 4.24), (1972, 4.40), (1973, 4.64), (1974, 4.65), (1975, 4.62), (1976, 4.89), (1977, 5.03), (1978, 5.08), (1979, 5.36), (1980, 5.29), (1981, 5.12), (1982, 5.08), (1983, 5.07), (1984, 5.24), (1985, 5.41), (1986, 5.60), (1987, 5.73), (1988, 5.95), (1989, 6.07), (1990, 6.10)
Experiments
1. Preliminary Effects of Fossil Fuel
Emissions
We will run the first experiment with the
basic model, described above, to see how the d 13C
of the atmosphere will change, recognizing that this is a fairly primitive
model at this point. Set the program to run from 1860 to 1990 with a time step
of 1.0, using the Runge-Kutta 2 integration method. Before running the model,
pause to make a prediction. What will happen? How much (and in what direction)
will the atmospheric d13C change? Analyze the results in the form
of a graph that shows d13C for the atmosphere, the total carbon in
the atmospheric reservoir, and the FFB flow. How realistic are the results? You
can consider the results in a qualitative sense and also in a quantitative
sense by comparing the actual record, seen in Figure 7.02, with the model
output.
2. Reducing FFB by 50%
Part of the problem with our first model is
that all of the carbon produced by FFB stays in the atmosphere. In reality,
only about 50% seems to be accumulating. Let's assume that, over the course of
the last 130 years, this 50% retention figure has remained the same. We can
implement this change by simply modifying the FFB flow so that it is equal to
0.5*FFB_history. It should be fairly easy to predict the outcome of this
change. How do the results compare with the known record of atmospheric d 13C? In making this change, we are implicitly assuming that
the mechanisms that remove some of the carbon from the atmosphere remove carbon
with the same d 13C value as the carbon produced by fossil
fuel burning. This assumption is probably wrong and we'll address this issue in
the next experiment.
3. Adding Outflows -- Exploring the
Problem of the Missing Sink
Now we'll make another increase in complexity
by adding two outflows that represent the main sinks for atmospheric carbon --
uptake by the ocean and photosynthesis by land plants. Together, we will assume
that these flows account for 60% of the carbon released by FFB. Both of these
processes also involve an isotopic fractionation -- they prefer the lighter
form of carbon. Photosynthesis, as we mentioned before, involves a -25‰ shift
or fractionation, which means that the carbon it extracts from the atmosphere
is always 25‰ lighter than the d13C of the
atmosphere. The transfer of carbon from the atmosphere to the sea involves a
smaller fractionation, of around -2‰, again showing a preference for the
lighter form of carbon, which can diffuse across the air-sea boundary more
easily. This is complicated by the fact that the reverse process -- the
transfer of carbon from the sea to the air -- involves a different
fractionation (around -9 to -10‰). But in our model here, the ocean will not be
permitted to give carbon to the atmosphere, so we will ignore this process.
The question now is how to incorporate these
carbon uptake processes that involve fractionation. Let's begin by examining
our basic equation that we developed above;
![]()
Here, the outflow process is represented by Fo,
and the carbon it removes is characterized by a ratio of do. But, in this case, do is not a fixed value -- it depends on dM, the ratio of carbon in the reservoir that is being
drained. In other words,
![]()
where Do is the
isotopic shift (or fractionation), such as -25‰ for photosynthesis. Then if we
plug (7) into (6), we end up with:
![]()
which can be expanded to include the effects
of two outflow processes with separate isotopic shifts, to give us the final
equation we need for our enhanced model:
![]()
Incorporating these changes leads to a
revision of the model, shown in Figure 7.19. Below, I list the equations (minus
the graph of the history of fossil fuel burning) of this modified model.
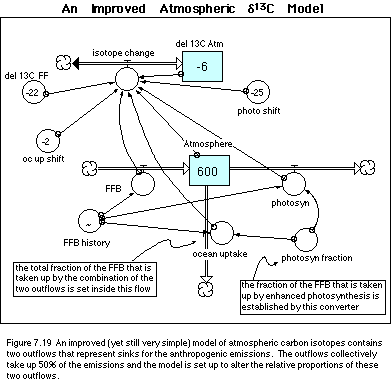
· Equations for an Improved Atmospheric d13C Model
Reservoirs:
INIT Atmosphere = 600 {Gt C}
INIT del_13C_Atm = -6 {pre-industrial del13C}
Flows:
FFB = FFB_history {FFB stands for fossil fuel burning}
photosyn = FFB_history*photosyn_fraction
ocean_uptake = FFB_history*(.5-photosyn_fraction)
isotope_change = (1/Atmosphere)*((FFB*(del_13C__FF-del_13C_Atm))-(photosyn*photo_shift)-(ocean_uptake*oc_up_shift))
Converters:
del_13C__FF = -22 {del13C of fossil fuels}
photo_shift = -25 {isotopic shift for photosynthesis}
oc_up_shift = -2 {isotopic shift for air-to-sea transfer}
photosyn_fraction = .25 {fraction of FFB taken up by enhanced photosynthesis}
FFB_history = same as first model
With this improved, more realistic model, we
can venture into the realm of paying closer attention to the quantitative
results of the model, although we must remember that we are still dealing with
only a reduced version of the global carbon cycle. This model provides a means
of exploring some possible solutions to one of the major questions concerning
the global carbon cycle -- the problem of the "missing sink". At the
present time, we cannot really balance the budget of the carbon cycle. We know
that about half of the FFB emissions are accumulating in the atmosphere, and
the available evidence indicates that around 20% to 30% is being swallowed up
by the oceans, leaving another 20% to 30% that must be going somewhere -- into
the "missing sink". Most people assume that the terrestrial part of
the carbon cycle makes up the missing sink, but it is extremely difficult to
accurately measure, on a global scale, where this extra carbon is going. With
this model, we can explore a couple of possibilities, outlined below.
1) What if all the excess carbon -- 50% of FFB -- is taken up by the oceans with none of it going into the terrestrial biosphere? What are the implications for the atmospheric d13C? Do the observations rule out this possibility? Can we alter the ocean uptake isotopic shift so that the model results come into close agreement with the observations? (We're hoping to end up in 1990 with an atmospheric d13C of around -8 to -8.5.) How much do you have to change this isotopic shift to get the model to fit the results?
· 2) What if all the excess --50% of FFB -- carbon is taken up by the terrestrial part of the carbon cycle? What are the implications for the atmospheric d13C? Do the observations rule out this possibility? Can we alter the photosynthesis isotopic shift so that the model results come into close agreement with the observations? How much do you have to change this isotopic shift to get the model to fit the results?
3)
If we preserve the isotopic shifts at -2‰ and -25‰, what mix of ocean uptake
and photosynthetic uptake gives us an atmospheric d 13C of around -8 to -8.5 by the year 1990?
Exploring the Dynamics of the
Cretaceous-Tertiary Crisis
Background on the K/T Mass Extinction
In this section, we will apply many of the
same ideas developed above to investigate one of the most important events in
Earth's history -- the mass extinction that occurred at the Cretaceous-Tertiary
(K/T) boundary, 65 million years ago. At this time, something like 50% of the
known genera (and perhaps 80% at the species level) of organisms became
extinct, including the dinosaurs. The extinction of the dinosaurs is an
interesting case since, as a group, they had done very well for a very long
time -- they were a great success story, and then they disappeared. But more
importantly for the biosphere and the whole Earth system, many other organisms,
including most species of plankton, also became extinct, thus dealing a serious
blow to food webs in many ecosystems.
This mass extinction effectively
"cleared the slate" biologically, and in the aftermath, the few
survivors were faced with a very different world, one with all sorts of
possibilities and unoccupied niches. All of this opportunity is thought to have
encouraged prolific speciation -- the evolution of new life forms. Mammals had
been around in small numbers for many millions of years before the K/T
boundary, but they never seemed to be capable of really establishing themselves
in a wide variety of niches. But, some of the mammals survived the K/T mass
extinction and in the aftermath, they underwent an evolutionary explosion with
many new species evolving, one of which eventually led to us. So, in a sense,
part of the reason we are here today is that mammals happened to survive the
K/T mass extinction.
Perhaps the central question in this whole
matter is: what caused this mass extinction? Understanding the time over which
the extinctions took place is another key question and it is this question that
ultimately led to our present understanding of what caused this crisis. Before
1980, this matter of the cause received plenty of attention, but most of the
ideas were highly speculative -- no one had really found any good hard evidence
to support any hypothesis.
Iridium and the Impact Hypothesis
Around 1980, Walter Alvarez and some
colleagues were studying a sequence of rocks in Italy, studying the reversals
of the magnetic field that were recorded in these rocks. Walter happened to
notice that in these rocks, which were deposited on the floor of a deep sea,
that the K/T boundary was marked by the very sudden disappearance of planktonic
fossils. A thin clay layer, 3 cm thick, separated the Cretaceous from the
Tertiary and just below this clay layer, there were plenty of Cretaceous
plankton, so the extinction looked to be very abrupt. But, knowing that a
bedding plane can sometimes represent a huge gap in time, Alvarez wondered if
there was some way of measuring how much time the clay layer represented --
that would provide a powerful constraint on the length of time over which the
extinction took place. Walter's father, a Nobel laureate physicist, came up
with an ingenious idea -- measure the concentration of iridium in the layer.
Iridium is very rare at the surface of the Earth (most of Earth's allotment of iridium
is deep in the core), but it does fall to the surface as dust particles from
micro-meteorites that burn up in our atmosphere. This rate of fall-out is
known, and so if you know the concentration, you can figure out how much time a
layer of sediment represents.
When they measured the iridium concentration
of this clay layer, they found that it was unbelievably high -- they had the
magnetic reversals to provide an upper limit on the length of time the clay
layer could represent, and the iridium seemed to exceed this upper limit by a
wide margin. Puzzled and intrigued, they sought another explanation for the
high concentration of iridium and eventually came up with the idea that the
iridium came from a very large asteroid that hit the Earth and effectively
delivered a huge slug of iridium all at once. Knowing the average concentration
of iridium in asteroids, they calculated that the asteroid was 10 km in
diameter. Asteroids travel at a velocity of around 15 km/sec2, which, combined with the mass of the object, would
produce an explosion on impact that exceeds the explosive energy of all the
nuclear weapons on earth by a factor of about 10,000! This explosion is
unimaginably large and it would have blown a huge crater in the Earth's
surface, blasting the vaporized, pulverized impactor and target rock high above
the troposphere, quickly forming a dense, opaque blanket over the Earth. This
impact cloud would have blocked the sunlight for at least several months,
plunging the Earth into an extremely cold state and halting photosynthesis,
which is the basis of the food chain. Hot droplets of molten rock would have
rained down on the surface, igniting fires. Shock-heating of the atmosphere
would have produced rainfall with an acidity approaching battery acid at least
in the region around the impact.
Testing the Impact Hypothesis
What a disaster! And what a story to come out
of such a thin, unassuming clay layer. But did it really happen? Are there
other pieces of evidence that can support or refute this hypothesis? One
prediction is that this iridium-bearing layer should be found all over the
globe, and it has in fact been located in several hundred localities, spanning
the globe. Another prediction is that there should be small fragments of the
target rock, tiny sand-sized particles that were blasted from the impact site
and settled into the same clay layer with the iridium. The origin of these sand
grains could be determined by the presence of rare features that form in quartz
crystals as a result of a shock wave passing through rocks. The only natural
way of forming shock-deformed quartz is in an asteroid impact, so these shocked
quartz grains are extremely helpful in testing the impact hypothesis. As it
turns out, these shocked quartz grains do occur in the clay layer. Another
thing found in the clay layer is soot that is thought to be from huge fires on
the continents; the amount of soot implies that up to one-half of the plant
material on land burned at this time.
In addition, the impact hypothesis predicts
that we might be able to locate the actual impact crater, which could then be
connected based on age and chemistry to the materials found in the clay layer.
And, once again, it turns out that such a crater has been found, buried beneath
hundreds of meters of younger sediment in the Yucatan Peninsula of Mexico, at a
site called Chicxulub.
Over the last 10 years, there has been a
nearly unprecedented frenzy of activity investigating this hypothesis and it
seems to have passed all the test with flying colors, and as a result, the vast
majority of geologists now favor this hypothesis as the best explanation for
what caused the mass extinctions at the K/T boundary. The realization that our
Earth has suffered such catastrophes came a shock (pardon the pun) to
geologists who previously had been conditioned to think of gradual, slow
changes occurring over vast spans of time. We now appreciate that dramatic,
rapid, even catastrophic changes do occur and they can be among the most important
events in the history of the Earth.
Modeling the K/T Impact Crisis
The K/T crisis presents some very interesting
opportunities for understanding how the Earth system responds to very extreme
perturbations. Does it recover and return to a steady state? The fact that we
exist tells us that it certainly does recover, but how quickly?
There are several approaches that we can take
to understand how the various parts of the system respond to this kind of a crisis.
We could investigate the effects using our simple climate models developed an
earlier chapter on climate modeling. Or we could investigate it by altering the
global carbon cycle that we developed in the first section of this chapter. I
encourage you to return to those models and think about how you would modify
them to represent the changes imposed by the dust cloud of the impact. One
obstacle to this modeling approach is that we would need to make some
assumptions about how the biosphere reacted to the crisis. For instance, how
long did photosynthesis stop? How greatly was the ocean biologic pump affected,
and for how long? To get at some of these questions, we can use a model of
carbon isotopes in the ocean-atmosphere system to help us interpret the meaning
of the measured carbon isotopes from planktonic and benthonic (bottom-dwelling)
organisms. This will help us understand what the effects of the impact actually
were on the ocean system.
First, let's look at the actual data that has
been acquired from deep-sea sediments spanning this event. Figure 7.20 shows
the general story, as told by organisms living in the surface waters and the
bottom of the oceans. These organisms make their shells out of calcite and the
calcite represents a sample of the sea water where the organisms were living.
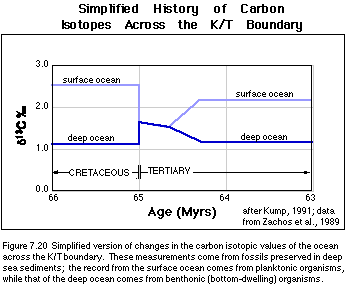
Looking at Figure 7.20, we can see that before the impact, there is the expected difference between the surface ocean and the deep ocean, with the surface having more positive d13C values than the deep ocean because of the operation of the biologic pump. Then, just after the impact, the d13C of the surface water plummets, and the d13C of the deep ocean rises slightly until the two are more or less equal; then begin a slower decline together until about 500 Kyr after the impact, when the two diverge again. The period of time when the surface and deep ocean d13C values are the same represents what is called a "Strangelove" ocean (i.e., the post-apocalyptic ocean) after the Peter Sellers character in Stanley Kubrick's outstanding movie titled Dr. Strangelove. From this isotopic record of what happened, we would like to be able to say something more specific about what happened to the biologic pump. To do this, we need to develop an ocean-atmosphere carbon isotopic model.
Constructing the Model
This model will be based on the simple ocean
- atmosphere carbon cycle model represented in Figure 7.13, with several
modifications to help us keep track of the organic carbon and inorganic carbon
(carbonate) moving between the surface and deep ocean reservoirs. The reason
for doing this is because of a fractionation that occurs when organisms fix
carbon from sea water to make their soft parts; this makes the organic carbon
much lighter, shifted by -25‰, while the inorganic carbon will simply be the
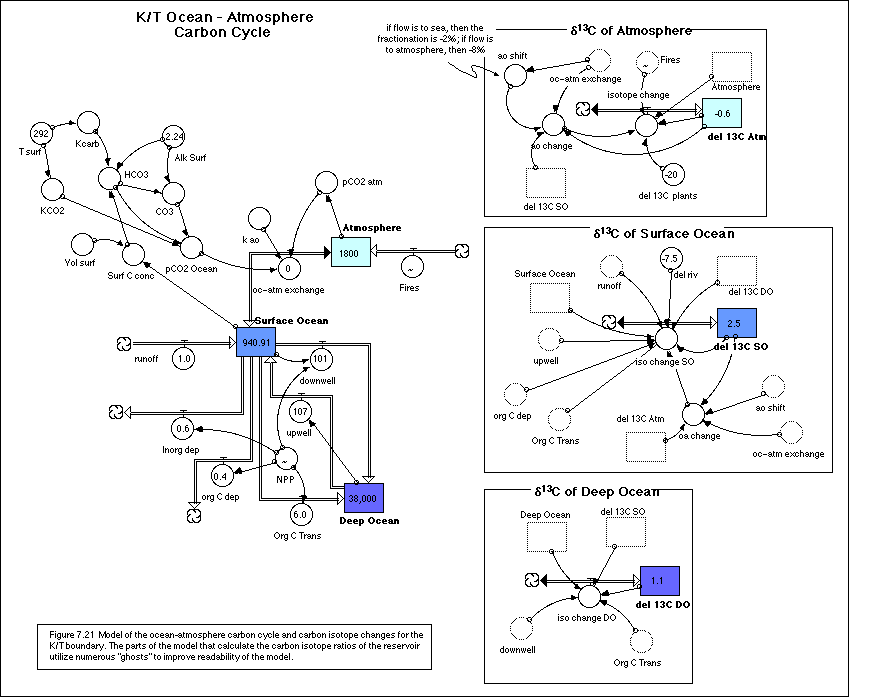
same as the sea water d13C. Figure 7.21 shows the structure of this
model, divided up into different zones; one zone models the mass flows of
carbon (in gigatons), while the other zones model the changes in isotopic
ratios of the carbon stored in the three different reservoirs.
· Equations for K/T Ocean-Atmosphere d13C Model
·
Reservoirs (mass of carbon):
INIT Atmosphere = 600 {Gt C}
INIT Deep_Ocean = 38000 {Gt C}
INIT Surface_Ocean = 884.391 {Gt C}
Reservoirs (isotopic ratios of carbon):
INIT del_13C_Atm = -6 {pre-industrial del13C}
INIT del_13C_SO = 2.5
INIT del_13C_DO = 1.1
Flows(mass of carbon):
fires = GRAPH(time)
(0.00, 0.00), (0.5, 0.00), (1.00, 0.00), (1.50, 0.00), (2.00, 0.00), (2.50, 0.00), (3.00, 0.00), (3.50, 0.00), (4.00, 0.00), (4.50, 0.00), (5.00, 0.00)
oc--atm_exchange = k_ao*(pCO2_atm-pCO2_Ocean)
downwell = ((1*NPP/8)+(140.2/1.4))*(Surface_Ocean/INIT(Surface_Ocean))
upwell = (150/1.4)*(Deep_Ocean/INIT(Deep_Ocean))
runoff = 1
inorg_dep = .6*NPP/8 {carbonate sediment deposited on the sea floor and buried}
org_C_dep = .4*NPP/8 {organic carbon deposited on the sea floor and buried}
org_C_Trans = 6*NPP/8 {organic carbon transferred to deep ocean, but not buried}
Flows (isotopic ratios of carbon):
isotope_change = (1/Atmosphere)*((Fires*(del_13C__plants-del_13C_Atm))+ao_change)
iso_change_DO = (1/Deep_Ocean)*((downwell*(del_13C_SO-del_13C_DO))+(Org_C_Trans*(del_13C_SO-25-del_13C_DO)))
iso_change_SO = (1/Surface_Ocean)*((runoff*(del_riv-del_13C_SO))+oa_change+(upwell*(del_13C_DO-del_13C_SO))+((org_C_dep+Org_C_Trans)*25))
Converters (masses of carbon):
Alk_Surf = 2.24
CO3 = (Alk_Surf-HCO3)/2
HCO3 = (Surf_C_conc-SQRT(Surf_C_conc^2-Alk_Surf*(2*Surf_C_conc-Alk_Surf)*(1-4*Kcarb)))/(1-4*Kcarb)
Kcarb = .000575+.000006*(T_surf-278)
KCO2 = .035+.0019*(T_surf-278)
k_ao = .278 {Gt C/yr/ppm -- the observationally-derived rate constant; this is for the entire surface area of the ocean}
pCO2_atm = Atmosphere*(280/600)
pCO2_Ocean = 350*KCO2*(HCO3^2/CO3)
Surf_C_conc = (Surface_Ocean/12000)/Vol_surf {1e18 moles/m^3}
T_surf = 288 {°K}
Vol_surf = .0363 {units are 1E18 m^3 -- this is the upper 100 m}
NPP = GRAPH(time {Gt C/yr net primary production -- net carbon extracted from sea water by organisms})
(0.00, 8.00), (10.0, 8.00), (20.0, 8.00), (30.0, 8.00), (40.0, 8.00), (50.0, 8.00), (60.0, 8.00), (70.0, 8.00), (80.0, 8.00), (90.0, 8.00), (100, 8.00)
Converters (isotopes of carbon):
del_13C__plants = -20 {del13C of fossil fuels}
del_riv = -7.5 {avg isotopic value of carbon delivered by rivers}
ao_shift = if oc--atm_exchange>0 then -2 else -9.5 {the isotopic shift of the ocean-air transfer depends on the direction of flow -- data from Siegenthaler and Munnich, 1981}
ao_change = if oc--atm_exchange>0 then -oc--atm_exchange*ao_shift else oc--atm_exchange*(del_13C_SO+ao_shift+del_13C_Atm) {net effect of the ocean-air transfer}
oa_change = if oc--atm_exchange>0 then oc--atm_exchange*(del_13C_Atm+ao_shift-del_13C_SO) else -oc--atm_exchange*ao_shift {net effect of the ocean-air transfer}
In the carbon mass portion of the model, the
flow that was previously called the biologic pump is here broken up into four
different flows, which are all fractions of the net primary production, NPP.
The NPP here represents all of the carbon, organic and inorganic, fixed by
organisms living in the surface oceans. The model is initially set up with an NPP
of 8 Gt C/yr; of this, 6.4 Gt is organic carbon, 6 of which is decomposed by
microbes and added to the water of the deep ocean, while 0.4 Gt is eventually
deposited and buried in sediments on the sea floor. The 1.6 Gt C or carbonate
(inorganic carbon) is divided up into 1 Gt (added to the downwelling flow) that
gets dissolved on its way into the deep ocean, and 0.6 Gt that is deposited and
buried on the sea floor.
The initial reservoir values in this model
are intended to approximate the conditions at the end of the Cretaceous, when
the Earth was significantly warmer. The atmospheric reservoir was set so that
the CO2 concentration of the
atmosphere is three times the present value. The temperature of the surface
waters of the oceans was increased by 4°, and then the carbon content was
adjusted until the CO2
concentration matched that of the atmosphere. The isotopic values of the
oceanic reservoirs are based on measurements from the shells of organisms, while
the isotopic value of the atmosphere, for lack of measured values, is set to
the pre-industrial value of -6‰.
The isotopic portion of the model is
complicated by the fractionation that occurs when CO2 is exchanged between the atmosphere and the ocean. If
CO2 moves from the atmosphere
to the ocean, there is a -2‰ fractionation, but if it moves the other way,
there is a -8‰ fractionation. In the model, this necessitates an
"if-then-else" statement.
Experiments
1. Sudden, Complete Killing
What happens if we kill off all the organisms
in the surface waters, disabling the biologic pump, sending the NPP to 0? More
precisely, the questions to ask are: 1) what happens to the carbon cycle as a
result of this change?; and 2) how is this change reflected in the carbon
isotopes of the ocean reservoirs?. To simulate this sudden, complete killing,
change the graph of the NPP so that it is equal to 0 rather than 8 for the duration
of the experiment. Make predictions and then run the model for 100 years with
time step of 0.1 using the Runge-Kutta 2 method of integration. After studying
the short-term effects, run the model out for 2000 years (this will take a few
minutes). You may find it helpful to plot the surface and deep ocean d13C values at the same scale, to facilitate
comparison with the observed record shown in Figure 7.20.
2. Varying the Magnitude and Duration of
the Kill
In the above experiment, we killed off
everything in the oceans (at least as far as the carbon cycle is concerned) and
we kept the oceans in this dead state for the duration of the experiment.
Comparing the results from experiment with the observed record shows a fairly
good agreement, making us think that perhaps the oceans really were effectively
dead for quite a long time. But, we might wonder about how severe of a killing
is needed to show up as a noticeable change in the isotope record. By
"severe" I mean the magnitude of reduction in the biologic pump, and
the duration of this reduction. In order to alter the method for defining the
severity of the killing or reduction in NPP in the oceans, we will add four new
converters to the model, following the scheme described in Figure 7.22.
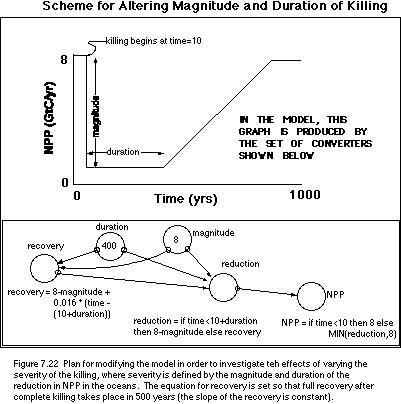
With this new scheme in place, we can readily
change the magnitude and duration of the killing to see how the isotopic values
of the ocean reservoirs respond. The primary question to investigate here is
what is needed in terms of magnitude and duration to make the surface and deep
ocean d13C values equal, as is observed in the
post-K/T Strangelove ocean.
3. Effect of Large-Scale Global Fires
If you recall from the above brief discussion
of the soot found in the K/T clay layer, it appears that a significant portion
of the land plants burned; this would have released a great deal of light
carbon into the atmosphere. What happens to the carbon cycle and especially the
carbon isotopes if we include this proposed large-scale burning that took place
after the impact (fires ignited by some of the super hot fallout of the
impact)? We can represent these fires by modifying the graph that appears
inside the Fires flow. On this graph, which relates the Gt of carbon released
by fires over time, we can create a scenario of burning and observe its
effects. Let's imagine that the burning is fast and furious beginning at
time=10, releasing about 100 Gt/yr, then returning to 0 by time=14. To clearly
understand the effect of these fires on the quantities of carbon and the d13C values of the major reservoirs, run
this model for about 100 years with no reduction in the NPP. Then, after you
have studied the results and understand why the model behaved as it did,
combine this same fire-burning scenario with a scenario of complete killing and
compare the results with a model in which there are no fires -- this will allow
you to see if the fires might have had a significant effect on the d13C values of the oceanic reservoirs in the
aftermath of the K/T impact crisis.
Summary of Carbon Isotope Modeling
In this section, we extended our modeling of
the global carbon cycle to investigate the changes in the ratios of carbon
isotopes that accompany changes in the distribution of carbon in the global
cycle.
The carbon dioxide emitted to the atmosphere
by fossil fuel burning has a very different isotopic makeup than the
atmospheric reservoir as a whole, thus we can see the human effects on the
carbon cycle not only in the increasing amount of carbon in the atmosphere, but
also in the changing ratio of carbon isotopes in the atmosphere. The observed
record of atmospheric d13C also provides a constraint on the problem of the
missing sink -- the problem of where all of the anthropogenic carbon dioxide is
going (only 50% stays in the atmosphere).
We also developed a model of the ocean-atmosphere
carbon cycle and associated isotopic changes to investigate what happened at
the K/T boundary, one of the most important climatic and biotic rises in
Earth's history. The model allows us to more clearly interpret the record of
carbon isotopic changes in the oceanic realm following the impact. This crisis,
which led to the extinction of around 80% of all species appears to have dealt
a major blow to the carbon cycle, but the system appears to have recovered
nearly completely within about 500,000 years. The climate just after this time
appears to have been more or less the same as just before this time. This
recovery of the carbon cycle and the climate system as a whole is a remarkable
feat and tells us something very important -- the whole Earth system is
surprisingly resilient on time scales of a million years or so. It has the
ability to recover and regenerate its stabilizing mechanisms.
It is important to keep the time scale of
recovery in mind when thinking about our present condition and the prospects
for our future well-being on this planet. If we do continue to tamper with the
climate and the biosphere, it is conceivable that we might cause a crisis that
makes the Earth system very inhospitable to us and many other organisms for a
very long time relative to our lifetimes, but in the long run, we have every
reason to believe that the Earth will recover (with or without our species).
Readings and Key References:
Broecker, W.S., and Peng, H.-S., 1993, Greenhouse Puzzles, New York, Eldigio Press, 251 p.
Gifford, R.M., 1993,
Implications of CO2 effects on vegetation
for the global carbon budget, in, Heimann, M., (ed.), The Global Carbon Cycle,
NATO ASI Series, v. 115, p. 159-200.
Holmen, K., 1992, The Global
Carbon Cycle, in, Butcher, S., Charlson, R., Orians, G., and Wolfe, G., (eds.),
Global Biogeochemical Cycles, London, Academic Press, p. 237-262.
IPCC, 1995, Climate Change
1994, Cambridge, Cambridge University Press, 339 p., but mainly p. 35-71.
Kump, L., 1991, Interpreting
carbon-isotope excursions: Strangelove oceans, Geology, v. 299, 302.
Kwon, O.-Y., and Schnoor, J.L.,
1994, Simple global carbon model: the atmosphere-terrestrial biosphere - ocean
interaction, Global Biogeochemical Cycles, v. 8, p. 295-305.
Post, W.M., Peng, H.-S.,
Emanuel, W.R., King, A.W., Dale, V.H., and DeAngelis, D. L., 1990, The global
carbon cycle, American Scientist, v. 78, p. 310-326.
Siegenthaler, U., and
Sarmiento, J.L., 1993, Atmospheric carbon dioxide and the ocean, Nature, v.
365, p. 119-125.
Walker, J.C.G., 1991, Numerical
Adventures with Geochemical Cycles, Oxford, Oxford University Press, 192 p.
