Nuclear power provides about 20% of the US electricity supply. To understand how it works we need to understand the nucleus of an atom.
We tend to think of atoms as solid balls linked together with chemical bonds forming molecules. The reality is that the "hard" shell that represents an atom is a representation of the electron that is whizzing around the central placed nucleus. The "solid" ball is a poor representation; instead think of a ping-pong ball. Most of the ball is empty; in the case of an atom the shell(s) (mass of electrons) does not contribute much to the mass, as electrons are very low in mass. The bulk of the weight is in the nucleus where the proton(s) and neutron(s) reside. These each have a mass of 1 atomic mass units (also called Daltons in the US). So hydrogen the simplest atom has 1 proton and 1 electron. It is the number of protons that determines the element so 1 proton is hydrogen represented as 1H. If there is also a neutron then the element is still hydrogen but it is a different isomer. We call these deuterium atoms and if we produce water from it we have "heavy water".
| Uranium 235 |
|
0.7 x 109 YEARS |
| Plutonium |
|
23,924,000 YEARS |
| Radon 222 |
|
3.8 days |
| Polonium 218 |
|
3 min |
There are many elements that are radioactive. For some, only certain of the isotopes are radionuclide (they are radioactive). However, some will have very long half-lives, others decay very quickly
INSC"
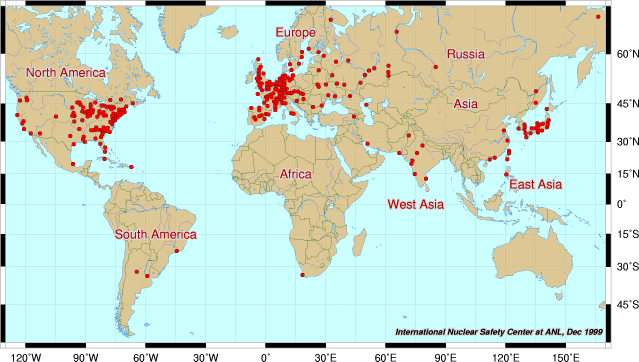
Nuclear Plants are scattered around the world, but its only the rich nations that can afford them! We are not happy that some "Axis of Evil" countries are joining the nuclear club.
We have a nuclear engineering degree at Penn State and a small nuclear reactor!

The nuclear reactor consists of a reactor with fuel rods, control rods and a moderator. The heat produces steam which is heat exchanged (so the water is not radioactive) before going through the turbine to turn the generator which produces electricity. A cooling tower helps to cool the low temperature steam before it is returned to the reactor. A containment structure prevents radioactivity from escaping.
Basically when the 235 U is induced into decay via capturing a neutron, the atom breaks apart violently producing 2 fragments that are the new atoms and several neutrons. Thus, when at the critical state 1 neutron is allowed to induce another 235 U to decay, while other neutrons are modulated such that they do not have the energy to induce decay. The fragments are moving quickly, they have lots of kinetic energy or in the case of atoms and molecules we can say they have a high temperature. Thus, we can create steam as discussed before to turn a turbine. This site is worth a quick browse: National Energy Institute. I also like this virtual tour.
We have about 104 reactors in the US producing electricity, far more reactors than any other single country! The oldest one running is the same age as I am! They are licensed for 40 years with a 20-year extension possible. As most were built in the 70's & 80's soon we are looking at dismantling a number of nuclear reactors.

